Clinical outcomes of instrumentation for spinal infection in elderly patients
Article information
Abstract
Objective
Spinal infection is a serious problem that is becoming increasingly common as the population ages. The mainstay of spinal infection treatment is proper antibiotic administration, and surgical management is considered if indicated. However, for various reasons, surgery in elderly patients is usually a challenge. The authors performed a retrospective study of operative cases of elderly spinal-infected patients in our hospital to evaluate the effectiveness, safety, and prognosis of spinal instrumentation.
Methods
Thirty-five patients (age > 65 years) with spinal infection underwent surgical treatment from 2012 to 2019 and were followed up for more than 1 year. They were divided into two groups: instrumented (group 1) or non-instrumented (group 2). We analyzed the characteristics of patients, clinical outcomes, and surgical data.
Results
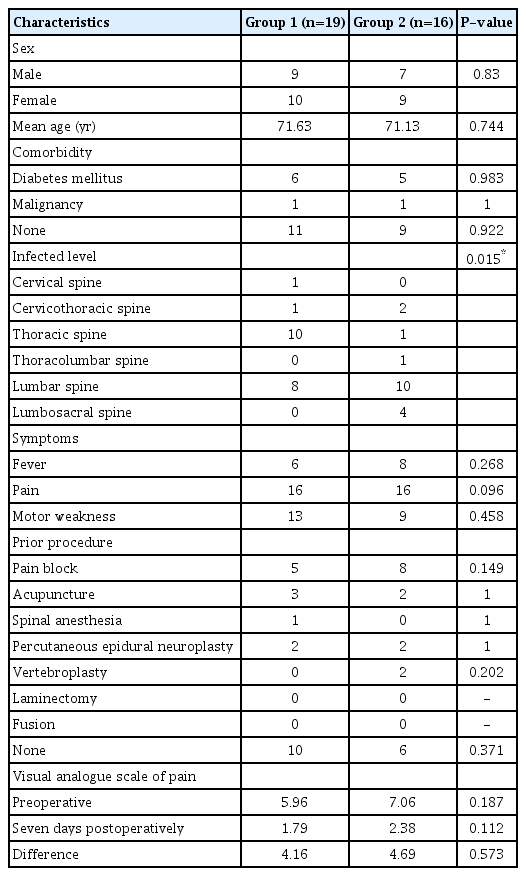
The preoperative characteristics, postoperative pain relief, improvement of infection, duration of antibiotic use, duration of hospitalization, and perioperative complications did not show statistically significant differences between groups 1 and 2. Spinal infection recurred in one patient in group 1 and five patients in group 2. The recurrence rate was significantly lower in the instrumented group (P = 0.042). The mean period from surgery to ambulation was 6.21 days (range, 1–23 days) in group 1 and 12.56 days (range, 2–34 days) in group 2 (P = 0.049).
Conclusion
Instrumentation to elderly patients did not increase the recurrence of infection or the incidence of significant complications in this study. Decompression with instrumentation is a reasonable and effective option for managing spinal infection in elderly patients.
Introduction
Despite increases in economic status, quality of life, and sanitary conditions, spinal infection remains a serious problem that can be life-threatening.
Spinal infection markedly reduces the quality of life because it can lead to pain, neurological deficits (radiculopathy, myelopathy, etc.), deformity and side effects due to the long-term use of antibiotics [1]. Furthermore, the incidence of spinal infections has increased from 5.3/100,000 people per year in 2007 to 7.4/100,000 people per year in 2010 [2-5]. Along with increases in incidence, the risk factors for developing spinal infections have also increased. It is believed that the incidence of diseases related to the spine has increased and the resulting spinal procedures, intravenous drug use, immunocompromised patients, and the development of radiologic modalities, such as magnetic resonance imaging (MRI) [6]. Furthermore, as we become an aging society, both the degeneration of the spine and related infection are expected to increase more and more.
Currently, the main treatment for spinal infection is the administration of appropriate antibiotics for pathogenic bacteria and surgical management is generally considered for neurological deficits, spinal instability, intractable pain, or if the infection is not controlled by medical therapy [7]. In principle, surgical management includes debridement, lavage, drainage of the infective lesion, and instrumentation, if needed. Instrumentation may be considered when spinal instability is present or instability is a concern after surgery. However, there is a variety of opinions concerning instrumentation for spinal infections because of the risk that instrumentation itself may become another focus of infection, leading to an extension of the surgical field and/or an increase in the surgical time. Some authors have stated that it is better not to insert instruments, even if surgical management is chosen as the treatment modality [8,9]. Other authors reported that surgical instrumentation can be covered with bacterial biofilm, so postoperative antibiotics don’t work on the infective lesion effectively [10,11]. Other authors have reported that surgical instrumentation improved postoperative instability and did not increase the recurrence rate of spinal infections [12-16]. Actually, study of elderly patients is rarely done and there is no definitive clinical consensus on the use of instrumentation for spinal infections.
Therefore, we performed a retrospective study of operative cases of elderly patients with spinal infections in our hospital to evaluate the effectiveness, safety, and prognosis (including recurrence of infection), etc., associated with the use of instrumentation. We believe that our review and conclusions will be helpful in therapeutic decisions to use instrumentation in elderly patients.
Material and Method
Patient selection
This retrospective study was conducted on patients (age>65 years) who underwent surgical treatment for spinal infections at our hospital from January 2012 to December 2019. Surgery was performed if antibiotic treatment failed. Among them, if there was a marked deformity, severe motion tenderness, or massive debridement for removal of infected tissue, additional instrumentation was performed. All patients were admitted by a single neurosurgeon and consulted by a single infectious disease physician. After surgery, they had a follow-up period of at least 12 months and clinical symptoms were assessed and serial laboratory tests, imaging, and physical examinations were performed. Patients with infections within 1 month of surgery and those with tuberculosis infections were excluded from the study. Due to retrospective design, this study does not require informed consent or the Institutional Review Board’s approval.
Patient and clinical assessment
Age, sex, neurological status, visual analogue score (VAS), and C-reactive protein (CRP) value were evaluated retrospectively. Neurological status was assessed preoperatively and postoperatively until the last follow-up using the manual muscle test. A motor power grade 0 represented complete paralysis and grade 5 represented normal power with a full range of motion against gravity and full resistance.
Imaging study
Evaluations were made by plain radiography (X-ray), computed tomography, and MRI. Specifically, MRI was used to confirm the spinal infective lesion causing neurological symptoms and to determine the level of surgical management. After surgery, X-ray was used periodically to diagnose postoperative kyphotic change and instability.
Surgical procedures
Surgery was performed if an antibiotic therapy failed or the patients had a neurologic deficit, intractable pain, or deformity. If a patient had a marked deformity, extensive removal of bone, spinal instability, or intractable axial pain, pedicle screw fixation was additionally performed for mechanical support (Fig. 1). The term instrumentation in this article means only screw fixation (Fig. 1). The operations were performed by a single surgeon.
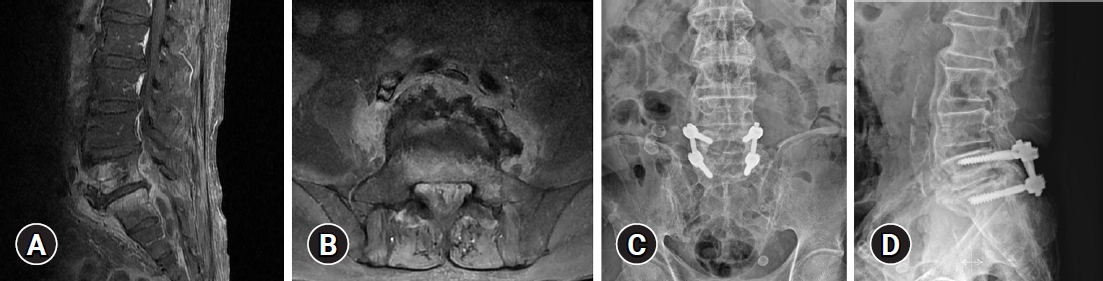
(A, B) Magnetic resonance imaging of a patient with pyogenic spondylodiscitis with epidural abscess and a postoperative X-ray after decompression, irrigation, and pedicle screw fixation. Infected tissue and an abscess were observed below L5. (C, D) We performed decompressive laminectomy, irrigation, and pedicle screw fixation.
Statistical analysis
The chi-square test was used for the analysis of categorical variables and the t-test for continuous variables. A P<0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA).
Results
In total, 35 patients were enrolled in this study. Among them, 19 patients (54.29%) underwent decompression with instrumentation (group 1). A total of 16 patients (45.71%) underwent decompression only (group 2). There were 16 male and 19 female patients and their ages ranged from 66 to 80 years with a mean age of 71.63 years in group 1 and 71.13 years in group 2. The characteristics of the enrolled patients are summarized in Table 1.
A total of 19 patients had various past histories. Eleven patients (31.43%; six in group 1 and five in group 2) had diabetes mellitus. Nineteen patients (57.14%; nine in group 1 and 10 in group 2) had a history of invasive pain procedures, including epidural block, acupuncture, and percutaneous epidural neuroplasty. Before the surgery, the most frequent symptoms were pain, such as tenderness or radiculopathy and was reported by 16 patients (84.21%) in group 1 and all in group 2. The VAS score decreased from 5.96 to 1.79 in group 1 and 7.06 to 2.38 in group 2 seven days after the operation. The differences were not statistically significant (Table 1).
Motor weakness due to an epidural abscess or destructed bone was seen in 13 patients (68.42%) in group 1 and nine patients (56.25%) in group 2. Ten patients (52.63%) in group 1 had thoracic infections, the most frequently infected site, whereas 10 patients (62.5%) in group 2 had lumbar infections. The difference between the two groups was statistically significant (P=0.015).
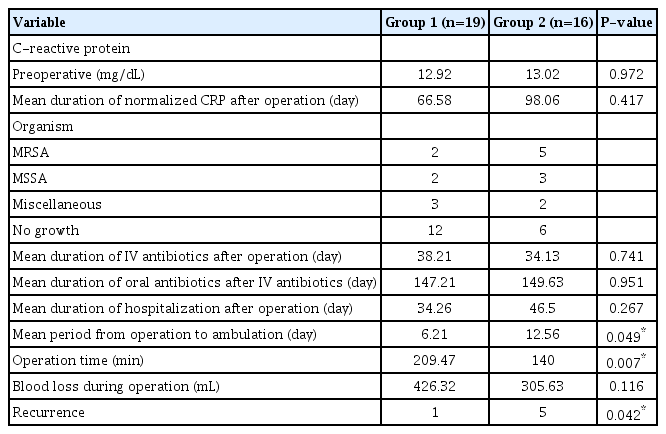
The mean duration of the normalized CRP after surgery was 66.58 days (range, 8−233 days) in group 1 and 98.06 days (range, 12−648 days) in group 2. Until normalization, CRP was measured through outpatient department as well as hospitalization. In 17 cases (48.57%), bacteria were identified. The most common bacteria were methicillin-resistant Staphylococcus aureus, in seven cases (41.18%; two in group 1 and five in group 2), followed by methicillin-sensitive Staphylococcus aureus in five cases (29.41%; two in group 1 and three in group 2). Before the bacterial identification and antimicrobial sensitivity tests, all patients were given intravenous antibiotics. The mean duration of intravenous antibiotic administration was 38.21 days (range, 10−208 days) in group 1 and 34.13 days (range, 11−76 days) in group 2 and the mean duration of oral antibiotics administered after the discontinuation of intravenous antibiotics was 147.21 days (range, 1−467 days) in group 1 and 149.63 days (range, 7−433 days) in group 2. The above values were not significantly different between the two groups and are summarized in Table 2.
The mean duration of hospitalization after surgery was 34.26 days (range, 12−81 days) in group 1 and 46.50 days (range, 13−180 days) in group 2. The mean period from surgery to ambulation was 6.21 days (range, 1−23 days) in group 1 and 12.56 days (range, 2−34 days) in group 2 (P=0.049). The patients with instrumentation could get ambulate earlier than non-instrumented patients and the difference was statistically significant (Table 2).
The mean operation time was 209.47 minutes (range, 120−395 minutes) in group 1 and 140 minutes (range, 70−225 minutes) in group 2 (P=0.007). The mean blood loss during surgery was 426.32 mL (range, 50−1,000 mL) in group 1 and 305.63 mL (range, 20−500 mL) in group 2. Three patients (one in group 1 and two in group 2) suffered from ileus after surgery.
Discussion
The principal treatment for spinal infection has been relatively well established. The administration of appropriate antibiotics and surgery, including irrigation, decompression, or fusion, if needed, are the chief treatments [1]. Surgical treatment is considered for patients with neurologic deficits, intractable pain, or unresponsiveness to medical treatment. The authors also treated patients with spinal infection, according to this principle. Even before that, such studies about instrumentation for spinal infection have often been reported about its efficacy, effectiveness, and safety. This study was done on elderly patients and, in that sense, we think this study is very meaningful.
In our results, instrumentation was performed in lumbar lesions more frequently than in thoracic or cervical lesions (P<0.05). Though the sample size of thoracic and cervical lesions was too small for adequate comparisons, the authors thought that it was due to the original hyper-movable characteristic of the lumbar spine. However, unlike our results, Baek et al. [17] reported that instrumentation for spinal infection was more frequently performed in thoracic or cervical lesions than in lumbar lesions. Specifically, if there were more than three involved levels, they reported that decompression alone was more frequent in lumbar or lumbosacral lesions. Because the treatment of spinal infection varies with the individual, further evaluation of this subject is needed.
In all cases of both groups, spinal infection eventually healed. Moreover, refractory fever or septic shock from systemic infection was controlled within three days after surgery. We think that it would not be from instrumentation, but from irrigation and debridement of infected tissue. Some authors who oppose instrumentation to infected bone have argued that instruments form a microbial film on the surface of the instrument. This biofilm can cause and maintain inflammation at the surgical site and cause infection to recur [18,19]. Nevertheless, in our study, there was no statistical difference in the CRP-normalization time between the patients who had decompression with instrumentation placement and the decompression-only patients. Spinal infection recurred in one patient in group 1 and five patients in group 2. The recurrence rate was lower in the instrumented group and the result was statistically significant (P=0.042). Regarding infection treatment, the use of instrumentation for spinal infection was not inferior to irrigation and debridement alone for the treatment of spinal infection.
Whether instrumented or not, the duration of both intravenous and oral antibiotics administration, the time to normalization of CRP, and the duration of hospital stays after surgery were not statistically significant. Many previous studies have reported on the efficacy and safety of instrumentation. However, most of the studies were done regardless of age. Our results for the elderly patients are also consistent with those studies [12-16,20-22]. The tendency toward efficacy and safety of instrumentation seen in the elderly patients in this study will be more supported and augmented by emerging techniques, such as antibiotics-coating and drug-delivery system [23,24]. The authors measured postoperative pain seven days after surgery. Whether instrumented or not, patient’s pain improved after surgery. Interestingly, the instrumented patients started ambulatory rehabilitation more quickly than the un-instrumented patients. Patients who underwent decompression only could not begin ambulatory rehabilitation until the pain improved. This is due to the excellent effect of instrumentation just after operation and that could encourage the patient to rehabilitation quickly. The disadvantage of prolonged bed rest and the advantage of rehabilitation with early ambulation have been made obvious by several studies, especially in elderly patients [25-27]. Although postoperative pain improved equally in both groups, if the immediate postoperative pain was improved enough to allow early ambulation for rehabilitation, the authors believe that instrumentation for spinal infection is a very reasonable treatment option.
The operative time in group 1 was longer than in group 2 because the instrumentation procedure was added. In our study, since it could not be determined whether perioperative complications resulted from prolonged surgical times, the prolonged surgical time was not considered to lead to postoperative complications. With such reason, three patients suffered from postoperative ileus.
Before the 2000s, several reports suggested the combination of autologous bone graft, debridement, and massive irrigation without instrumentation as the optimal surgical therapy for pyogenic spinal infection [28-31]. However, recently, surgical treatment with instrumentation for pyogenic spinal infection has been widely attempted and many authors have reported its acceptance as a therapeutic alternative [12-16,20-22,32-34]. When treating spinal infection with surgery, the author used instrumentation to reduce, not only motion tenderness from instability but also the risk of deformity progression. Although the sample size of both groups was small, our results demonstrated that instrumentation for spinal infection was not inferior to irrigation and debridement alone for the treatment of spinal infection.
Conclusion
In elderly patients, not only spinal infection, but also surgery for it can be said to be a challenge for the physician. Instrumentation to elderly patients did not increase the recurrence of infection without significant complications in this study. We believe that early ambulation and rehabilitation are very helpful in preventing complications in elderly patients who have undergone spinal instrumentation surgery. Decompression with instrumentation is a reasonable and effective option in managing spinal infection in elderly patients.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.