Delayed cerebral infarction due to vertebral artery dissection following a cervical spine fracture: a case report
Article information
Abstract
Traumatic vertebral artery dissection (VAD) following a cervical spinal injury is rare. Unilateral VAD does not usually cause neurologic symptoms, especially in the case of a non-dominant vertebral artery (VA), due to sufficient collateral blood supply. Nevertheless, doctors should be highly cautious of the possibility of new neurologic symptoms because dissection can cause symptomatic cerebral infarction after the latency period. An 80-year-old female patient visited the emergency room with posterior neck pain and dizziness that persisted since falling down the stairs. Immediately after the visit, a type 3 odontoid fracture including the C2 transverse foramen was identified, along with occlusion of the V2 segment of the left VA. No other abnormal findings were noted basilar top occlusion due to VAD occurred while planning cervical fixation surgery for the cervical fracture following the trauma without administering an anticoagulant. Mechanical thrombectomy was performed and internal trapping was performed for VAD. Subsequently, neurological symptoms showed improvement and the planned cervical fixation was subsequently performed. VAD must be suspected in some cervical fracture patients. Some reports have stated that administering intravenous heparin once VAD is identified may reduce the likelihood of a subsequent stroke; however, drug administration may inevitably be delayed in asymptomatic patients planning to receive additional surgery due to trauma. Cerebral infarction may occur due to a thrombus caused by the dissection at this time, but the cause may be eliminated with mechanical thrombectomy and internal trapping, which also obviates the necessity for continued antiplatelet administration.
Introduction
First described by Carpenter et al. in 1961, cervical spine fractures are reported in literature as a risk factor for vertebral artery injuries (VAI). The incidence of VAI in the literatures varies greatly, perhaps reflecting differences in study populations [1]. Asymptomatic unilateral vertebral artery dissection (VAD) does not usually cause neurologic symptoms and is treated with intravenous heparin [2]. However, when additional surgery is planned for a trauma patient, drug administration has to be delayed. Therefore, physicians should be highly cautious of the possibility of new neurologic symptoms because dissection can cause symptomatic cerebral infarction after the latency period.
Case Report
An 80-year-old female patient visited the hospital due to persistent posterior neck pain, left shoulder pain, and dizziness. She said that these symptoms began after falling 10 steps off the stairs at 5 pm the previous day. In the cervical spine computed tomography (CT) taken during the visit, fractures including odontoid process (type 3), spinous process, and transverse foramen of the C2 vertebra were identified (Fig. 1A, 1B). In the subsequently taken neck CT-angiography and magnetic resonance imaging (MRI)-angiography, a patent flow suspected to be retrograde flow of the V3 and V4 segments and total occlusion of the V2 segment were identified (Fig. 1C). In the subsequently taken cervical spinal MRI, findings of tear, partial tear, and contusion of the inter & supraspinous ligaments of the C2-3-4-5-6 were additionally identified; Therefore, internal fixation was planned for the earliest possible date once the condition had stabilized. Absolute bed rest was ordered while wearing Minerva orthosis cervical thoracic halo brace. As cervical spinal surgery was being planned and patent flow of right vertebral artery (VA) and total occlusion of left VA was identified, intravenous heparin was not administered for the traumatic VAD.
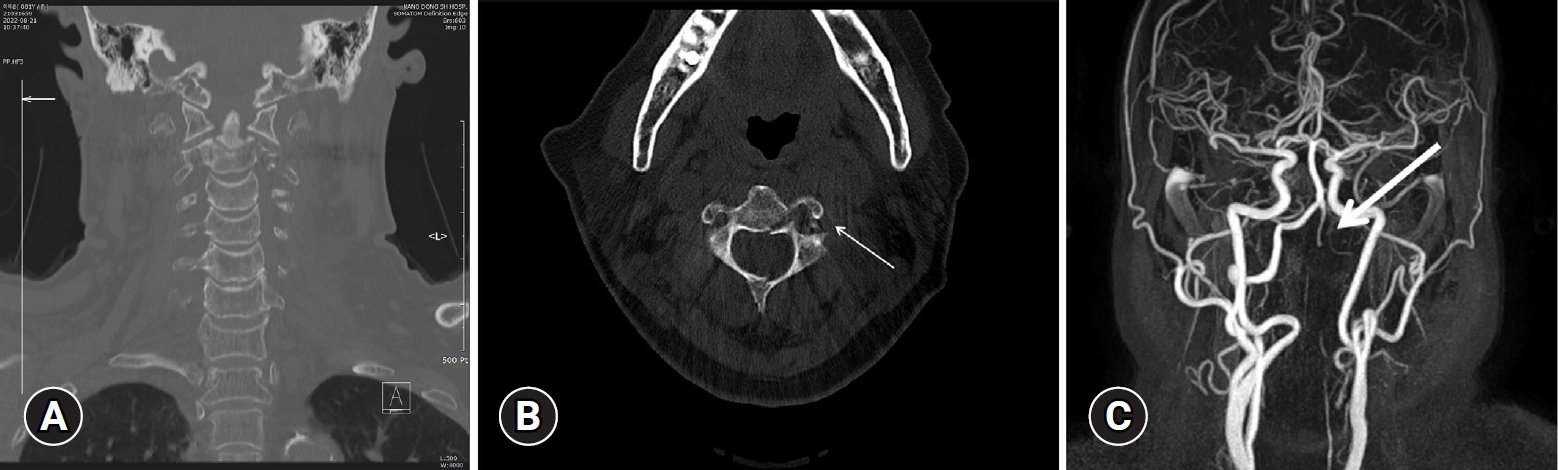
Imaging studies on admission. (A, B). Fractures including the odontoid process (type 3), spinous process, and transverse foramen of the C2 vertebra (arrow) were identified. (C) On magnetic resonance angiography, patent flow suspected to be retrograde flow of the V3 and V4 segments and total occlusion of the V2 segment (arrow) were identified.
Mild dysarthria occurred 7-day after the admission, but brain MRI diffusion-weighted image (DWI) did not show any abnormal findings. However, mental change to drowsy and dysarthria were accompanied again in the afternoon of the same day and brain MRI DWI was performed, which showed recent infarction in the left thalamus, and the left anterior choroidal artery territory (Fig. 2).
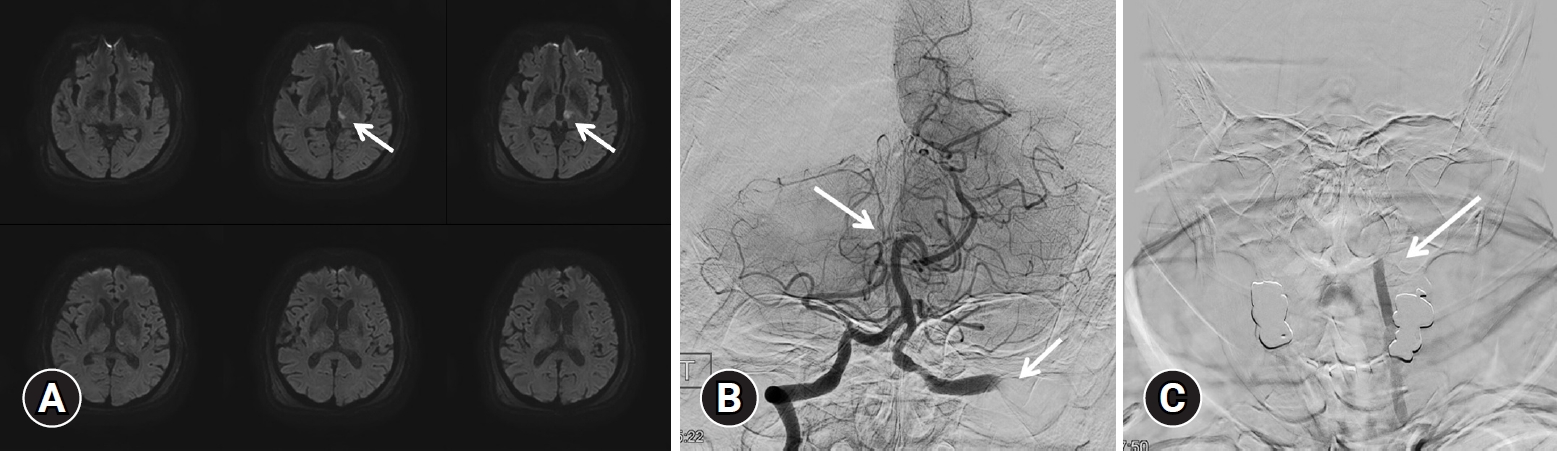
(A) Diffusion-weighted brain magnetic resonance imaging was performed, showing a recent infarction (arrow) in the left thalamus and the left anterior choroidal artery territory. (B) Angiographic image of the right vertebral artery. Occlusion of the basilar tip (arrow) and right posterior cerebral artery was identified. (C) Angiographic image of the left vertebral artery. Occlusion of the left V2 segment was identified (arrow).
Angiography was performed assuming that it occurred due to the thrombus in the dissection site. Occlusion of the left VA basilar tip and right posterior cerebral artery (PCA) were identified (Fig. 2B, 2C). A few days before the event, echocardiogram was performed to assess the surgical risk because additional surgery was scheduled. There were no abnormal findings like atrial fibrillation or thrombus in heart which meant the cardiogenic thrombus formation was less likely. Also, When during cerebral angiography, the patient's cerebral vascular condition appeared to be in good condition without any significant atherosclerosis. Therefore, it was estimated that the occurrence of right PCA occlusion occurred in gradual thrombus formation and migration after the left VA dissection. After performing aspiration twice using an aspiration catheter, recanalization of the thrombolysis in cerebral infarction scale IIb was confirmed (Fig. 3A). As cervical spine surgery was necessary due to the high instability with posterior ligament complex injury, the administration of anticoagulant and antiplatelet treatment was not feasible; therefore, internal trapping using a coil for the dissection was planned. Embolization using a coil was performed (Fig. 3B, 3C) and the complete control angiography confirmed that there was no flow to the V2, 3, 4 segments.
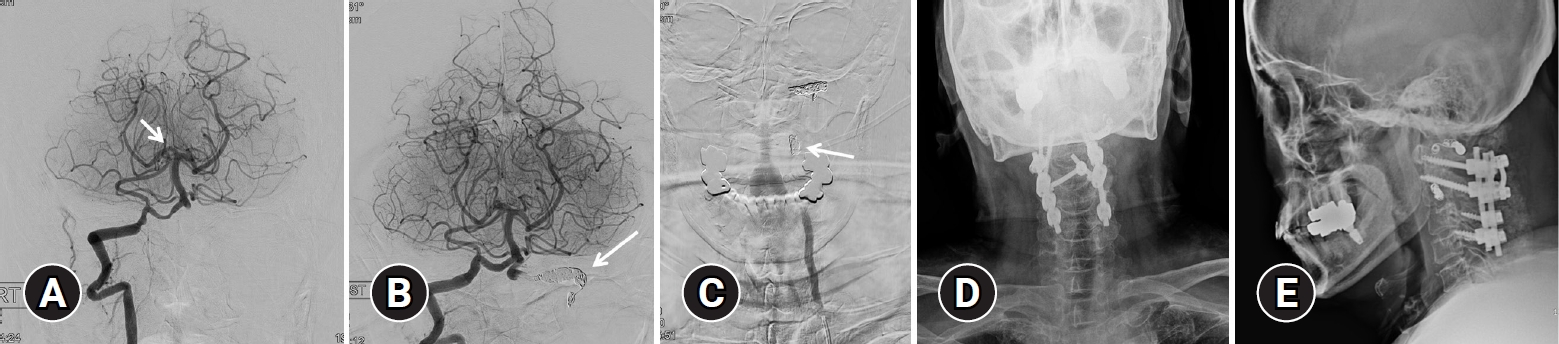
Angiographic findings and endovascular treatment of vertebral artery dissection. (A) After performing suction thrombectomy using an aspiration catheter, recanalization (thrombolysis in cerebral infarction scale IIb) (arrow) was confirmed. (B) Internal trapping using a coil for the distal segment of occlusion site (arrow) was performed. (C) Internal trapping using a coil for the proximal segment of the occlusion site (arrow) was performed. (D, E) Posterior cervical fusion was performed for stabilization.
After the surgery, it was confirmed that the symptoms were resolved to National Institutes of Health Stroke Scale 0. Subsequently, the patient was transferred to the stroke unit and aspirin and xanbon (ozagrel sodium) were administered for a week. In the brain MRI performed on the next day, additional abnormal findings other than procedure related embolic infarction were not identified. Bed rest was maintained under the use of the Minerva brace. Fever was checked and infection lab was elevated due to urinary tract infection. After 2 weeks of antibiotic treatment, the infection lab hae normalized. After that, posterior cervical fusion of C1-2-3-4 for stabilization was performed 18-day after the thrombectomy (Fig. 3D, 3E). After the surgery, the patient continued rehabilitation without specific findings and was discharged without neurologic symptoms. Persistent V2, 3, 4 segment occlusions were identified on the brain MRI performed 8-month after the acute infarction. This study was reviewed and approved by the Institutional Review Board of Kangdong Sacred Heart Hospital (IRB No. 2023-09-003). Informed consent was obtained from all individual participants included in this study.
Discussion
Traumatic VAD is rare following a cervical spine injury [3]. The incidence of VAD following cervical spine injury varies in the reports from 0.53% to 88%. This wide range of incidences of VAD may be due to small patient cohorts and inconsistencies in patient evaluation [4]. However, VAD may result in crucial consequences if unrecognized and untreated, with mortality rates up to 31% [5]. Therefore, screening for VAD is critical in suspected patients. The reason for screening of asymptomatic trauma patients for VAD is that it may occur in many asymptomatic patients and anticoagulation could prevent strokes and improve neurological outcomes [4].
There are no confirmed indications for immediate evaluation in a large study. However, some studies recommend immediate screening in the following cases [1,4]: The indication for immediate evaluation of the VA as follows: (1) fractures extending into the transverse foramen; (2) vertebral fractures with facet dislocation or pure ligamentous injury with facet dislocations; (3) vertebrobasilar ischemic symptoms following cervical spine trauma without obvious radiological signs of spine injury; and (4) no other life-threatening injuries.
The VA is most susceptible to injury especially at the point of entrance into the transverse foramen of C6. The second most common site is at C1–C2 [6]. Because of their course through several transverse foramen, the VAs are easily exposed to direct traumatic damage [7]. In addition, the probability of VAD according to fracture pattern varies and the incidence of VAI in fractures involving the transverse foramen is approximately 20% as in this case [3,4].
Giacobetti et al. [3] reported that flexion distraction-type injuries are the most common mechanism of blunt VA injuries, followed by a flexion compression mechanism. Fractures involving the transverse foramen may damage the artery within its bony structures. The initial injury to the artery most likely involves intimal disruption through excessive distraction and stretching of the artery between 2 adjacent transverse foramens [8].
Although digital subtraction angiography (DSA) is the gold standard for assessment of the cerebral vascular anatomy, CT-angiography and magnetic resonance-angiography are evolving as key imaging modalities in that they are noninvasive. With the increasing availability and accuracy, CT-angiography has widely replaced DSA as a first choice diagnostic tool for VAI in various centers. Several studies have reported a high accuracy of CT-angiography in detecting clinically significant injuries [9,10].
Nevertheless, even though VAD was identified through such screening, it is not easy to choose intravenous heparin for asymptomatic patients with trauma. Not only does anticoagulation in a patient with trauma causes a significant hemorrhagic risk, but there are cases where additional surgery is planned because of the trauma. During this period, delayed neurologic deterioration may be observed. A delay in clinical presentation has been attributed to propagation of distal extension of the dissection, mural thrombus into the basilar system, and embolization to the brain [2]. The site of dissection can serve as a site of progressive thrombosis or artery-to-artery emboli, the latter causing occlusion of the distal branch artery [11].
The treatment of acute VAD should be highly individualized. The optimal medical treatment of traumatic VAD is still under debate. Although evidence of anticoagulation in patients with VAD does not exist, until now anticoagulative treatment is a standard therapy. Previous studies showed a decreased stroke rate from 30%–50% to 2%–10% using anticoagulation or antiplatelet therapy [5,12,13]. Even in initial asymptomatic, untreated patients, the rate of neurological complications can be as high as 21% [14]. However, as mentioned above, anticoagulation in a patient with trauma causes a significant hemorrhagic risk and administration may not be an option when additional surgery due to trauma has been planned.
In such cases, interventional therapy could be an alternative once medical therapy fails or is contraindicated or symptoms of a vertebrobasilar embolism occur. Before the era of endovascular treatment, surgical trapping of a dissecting segment of the VA with or without bypass surgery was a curative treatment option; however, this operation has relatively high rates of morbidity and mortality [15]. One report has shown that internal trapping is a stable and effective treatment for acute VAD and that reconstructive treatment using a stent and coils could also be a feasible and safe alternative treatment modality. However, the outcomes of reconstructive treatment have not been well established [16].
Conclusion
In case of cervical spine fractures crossing the course of the VA, VAD should be highly suspected. When anticoagulation cannot be performed despite the diagnosis for VAD in trauma patients, caution must be taken for delayed cerebral infarction and treatment with internal trapping can be considered.
Notes
No potential conflict of interest relevant to this article was reported.
