|
 |
| J Korean Soc Geriatr Neurosurg > Volume 19(2); 2023 > Article |
|
Abstract
Objective
This study investigated the temporal dynamics of brain expansion following minimally invasive drainage for chronic subdural hematoma (CSDH) and explored potential influencing factors.
Methods
We retrospectively analyzed 101 patients diagnosed with symptomatic CSDH who underwent surgical treatment. The procedure involved twist drill trephination without irrigation, performed by a single surgeon, followed by catheter drainage. We measured subdural fluid volume before and after surgery, with catheter removal occurring 24 to 48 hours post-surgery. Monthly brain computed tomography scans were conducted for up to 3 months to monitor brain expansion. Outpatient follow-up was concluded after the volume increase plateaued. We examined the duration until follow-up termination, changes in subdural collection volume, and relevant medical records.
Results
On average, treatment termination occurred 148±43 days post-surgery. Notably, patient age positively correlated with treatment duration (Pearson coefficient=0.728, P=0.001). However, preoperative subdural hematoma volume, comorbid conditions (e.g., diabetes and hypertension), and initial drainage volume on the first post-surgery day did not exhibit significant correlations with treatment duration.
Conclusion
This study indicates that brain expansion following surgery for CSDH occurs, on average, at 148 days, and a positive correlation was identified between brain expansion and patients’ age. Given this finding, it is crucial to carefully schedule follow-ups for CSDH patients to safely determine or initiate interventions in cases of recurrence as necessary.
Chronic subdural hematoma (CSDH) is a condition in which the subdural space is occupied by hemosiderin-containing fluid, and the condition frequently develops following various intensities of trauma, especially in the elderly. Furthermore, the incidence of CSDH is expected to increase in the near future due to the aging global population and prevalent administration of antiplatelet and anticoagulation drugs among the general population [1].
Symptomatic patients with CSDH are indicated for burr hole trephination for drainage [2,3]. Even after the drain is removed, the majority of patients present with hematoma in the subdural space [4]. This occurs because it takes time for the brain to fully expand [5]. As a result, all such patients are recommended additional medical treatments such as angiotensin converting enzyme inhibitors (ACEIs) and statins [6,7]. Patients are also scheduled for brain computed tomography (CT) scans before termination of follow-up. Occasionally, subdural hemorrhage recurs, leading neurosurgeons to consider re-operation or long-term follow-up.
Remnant CSDH due to insufficiently expanded brain after surgery for CSDH suggests risk of recurrence. In patients with CSDH while receiving antiplatelet agents, cessation of the drugs is necessary; however, there are limited data on when to resume the medication and how long the follow-up period should last.
CSDH can be effectively treated with minimally invasive twist-drill craniostomy with subdural drainage. Saline irrigation during such operation is still debatable, however there currently are numerous literature in favor of minimal irrigation to eliminate potential traumatic effect of saline irrigation [8].
Patients showing remnant subdural hematoma postoperatively due to delayed expansion of the brain may require longer time before complete remission of CSDH. There currently is limited research on the duration of postoperative observation to sufficient brain expansion for subdural fluid collection to recede, as well as the factors that elongate the treatment duration.
The current study focused on the various potential risk factors and their correlation with the duration of follow-up period until complete remission of CSDH.
All patients who underwent twist-drill craniostomy with subdural drainage for CSDH between January 2012 and August 2022, under the care of a single surgeon, were included in this study. Patients who received bilateral twist-drill craniostomy with subdural drainage were counted as 2 separate cases.
Patients with incomplete postoperative assessments due to death, insufficient medical records, or loss of follow-up before confirming resolution were excluded from the data pool.
Each patient's medical records and radiographic examinations were reviewed to assess the modified Rankin scale score, medical history, CSDH-related symptoms, subdural hematoma volume on CT before and after surgery, postoperative medical therapy, and outpatient follow-up duration.
Patients with CSDH presenting various symptoms underwent surgery. The surgical procedure was uniform: twist-drill craniostomy with subdural drainage without irrigation, performed under endotracheal general anesthesia in a supine position by a single surgeon.
The management protocol is illustrated in Fig. 1. Postoperatively, a subdural drain is positioned at ground level to facilitate drainage. The medical team assesses the volume of hematoma drainage every 8 hours. Once the patient becomes asymptomatic or the drainage volume decreases to 10 milliliters per 8 hours, a follow-up CT scan is conducted, and if satisfactory, the subdural hematoma drainage catheter is removed.
The volume of CSDH is determined using brain CT images and a volume measuring tool within the institutional PACS system (Taeyoung Soft Co., Anyang, Korea). Patients are typically discharged four to 8 days post-surgery unless they have other medical issues requiring an extended hospital stay.
Follow-up brain CT scans are scheduled for one and 3 months after operation. If the patient is asymptomatic and there is no interval change in subdural hematoma volume on the last brain CT scan, follow-up is concluded, and the patient is considered to be in complete remission.
The population is stratified into 2 subgroups: the “immediate” subgroup comprises patients whose subdural fluid collection volume on the first postoperative brain CT scan is less than 50% of the preoperative brain CT scan, while the “delayed” subgroup encompasses patients with a subdural fluid collection volume on the first postoperative brain CT scan exceeding 50% of the preoperative brain CT scan. The subgroups were assessed on the modified Rankin scale score, medical history, CSDH-related symptoms, and outpatient follow-up duration to identify any factors correlated with prolonged duration of remission of CSDH.
All data are presented as mean±standard deviation. Univariate analysis of patient factors including age, gender, medical history, initial subdural hematoma volume was performed to assess impact on duration of follow-up period. Factors showing statistical significance in univariate analysis were further analyzed for Pearson correlation coefficient to determine their correlation with follow-up duration after twist-drill craniostomy with subdural drainage.
The statistical difference between subgroups in subgroup analysis is analyzed utilizing the Student t-test for continuous variables. Statistical significance is set at a value of P<0.05. For statistical analysis of the data, nonparametric correlations were determined using SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA).
A total of 115 patients who underwent twist-drill craniostomy with subdural drainage for CSDH between January 2012 and August 2022 was initially screened. Six patients were excluded due to incomplete medical records, and 8 patients were excluded due to death. Consequently, a cohort of 101 patients was included for medical record review.
The demographic characteristics of the cohort are summarized in Table 1. The average age of the group was 69±13 years. Underlying medical conditions were hypertension (n=45, 44.6%), diabetes mellitus (n=30, 29.7%), and hyperlipidemia (n=17, 16.8%). Patients presented with various symptoms, including cognitive dysfunction (n=20, 19.8%), headache (n=26, 25.7%), gait disturbance (n=11, 10.9%), dysarthria (n=10, 9.9%), hemiplegia (n=27, 26.7%), and altered mental status (n=7, 6.9%). The mean volume of subdural hematoma on the initial brain CT scan was 95±20.2 mL. The average duration of postoperative follow-up at the outpatient department was 148±43 days.
Univariate analysis was performed to assess potential impact of patient factors on the duration of follow-up period as shown in Table 2. Age of patient showed statistical significance (P=0.001).
There was a significant positive correlation between patient age and the follow-up duration until remission of subdural hematoma (Pearson coefficient=0.728, P=0.001), as illustrated in Fig. 2. However, there was no statistically significant correlation between subdural hematoma volume on the initial brain CT scan and the follow-up duration until remission (Pearson coefficient=0.117, P=0.244).
The patient characteristics by “immediate” and “delayed” subgroups are summarized in Table 3. The number of patients included in each subgroup was 43 in “immediate” subgroup and 58 in “delayed” subgroup. The “immediate” and “delayed” subgroups were also compared in mean age (67±15 and 70±11 years old, respectively), volume of subdural hematoma on initial brain CT (89.0±15.9 and 99.7±21.4 mL, respectively), and duration of follow-up until remission of subdural hematoma (147±48 and 148±40 days, respectively).
The duration of follow-up until remission of subdural hematoma in “immediate” and “delayed” subgroups were compared and results showed no statistically significant difference (P=0.066).
This study investigates the follow-up duration until remission in patients after CSDH surgery and identifies factors influencing this duration. The treatment of this condition includes medical, endovascular, and surgical therapies. However, there are disputes regarding the duration of the observational period and treatment due to the observed recurrence rate of CSDH, which can be as high as 70% [9].
CSDH is one of the most commonly encountered neurosurgical conditions, is particularly prevalent among the elderly population, and its prevalence is expected to increase in the future [10]. Symptomatic CSDH patients are recommended for twist-drill craniostomy with subdural drainage. Given its propensity for recurrence, CSDH is often treated with a combination of medical therapies, including steroids, statins, and ACEIs. Statins inhibit inflammation and immature angiogenesis, leading to rapid hematoma absorption, while ACEIs reduce the risk of CSDH recurrence by decreasing the concentration of vascular endothelial growth factor in the hematoma fluid, resulting in reduced vascular permeability [11,12]. Alongside medical therapy, patients with CSDH require vigilant observation periods since the condition can recur over varying durations [13].
Several factors contribute to recurrence of subdural hematoma, including previous use of antiplatelet agents, age, brain parenchymal atrophy, postoperative pneumocephalus, and preoperative types of CSDH [14,15]. Recurrence of CSDH is also thought to be related to delayed expansion of brain parenchyma due to an extended duration of brain compression by the hematoma [16,17]. The most common and universal cause of brain atrophy is aging, however there has been studies supporting that preoperative state of brain atrophy is not a statistically reliable predictive factor of recurrence of CSDH after burr hole trephination [5].
In this study, increasing age showed a positive correlation with the duration of follow-up until remission of subdural hematoma (Pearson coefficient=0.728, P=0.001). These data suggest that elderly patients with CSDH after minimally invasive subdural drainage require a longer watchful observation period compared to younger patients with the same condition. However, the initial volume of CSDH displayed no statistically significant correlation with the duration of follow-up (Pearson coefficient=0.117, P=0.244). As shown in Table 3, Subgroup analysis showed no significant difference between the “immediate” and “delayed” groups in mean duration of follow-up period (P=0.939). Thus these findings indicate that evacuating more than 50% of the initial volume of CSDH does not necessarily promote remission of the condition.
This study has several limitations. The indications for twist-drill craniostomy with subdural drainage may include subjective criteria, as only symptomatic patients were considered for surgical intervention. This study only included the volume of CSDH preoperatively. Additional information such as types, presence of septation, and Hounsfield units of CSDH is not collected as data. Such additional information might have further clarified correlating factors elongating the duration of treatment in patients with CSDH after surgery. Furthermore, immediate postoperative follow-up brain CT scans are performed at various time intervals, ranging from postoperative day 0 to 5. This variability may impact the postoperative evacuation percentage of subdural hematoma, potentially leading to misinterpretation of the effects of saline irrigation during the operation. Lastly, postoperative follow-up brain CT scans in the outpatient department are scheduled at three-month intervals, which could extend the duration of the follow-up period until CSDH remission.
The data analysis from this study reveals several key findings: (1) Increasing patient age necessitates a longer follow-up period for remission of subdural hematoma following twist-drill craniostomy with subdural drainage. (2) The initial volume of CSDH observed on the initial brain CT scan is not a reliable prognostic factor for estimating the duration of follow-up until CSDH remission. (3) The evacuation of more than 50% of the initial CSDH volume during twist-drill craniostomy with subdural drainage does not significantly impact the time to achieve remission.
Considering these results, it is advisable for neurosurgeons to anticipate longer follow-up periods after minimal invasive twist-drill craniostomy with subdural drainage in elderly patients. This study also suggests that the decision to terminate treatment in patients with CSDH following surgery should be based on clinical factors such as age and symptoms rather than solely on the absolute volume of CSDH on brain CT or the ratio of CSDH removed after surgery.
Fig. 1.
Treatment protocol for patients with chronic subdural hemorrhage. SDH, subdural hematoma; CT, computed tomography.
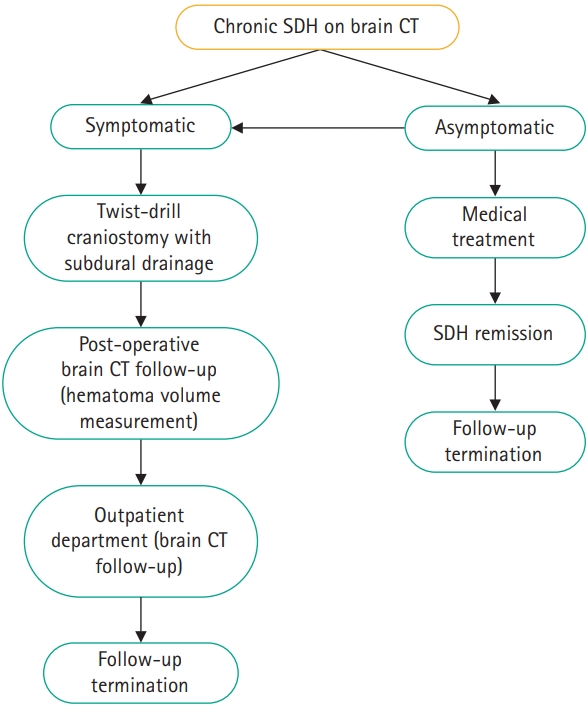
Fig. 2.
Scatter plot illustrating the correlation between patients’ age and the duration of follow-up until remission of subdural hematoma.

Table 1.
Patient characteristics
Table 2.
Univariate analysis of factors affecting follow-up duration
Table 3.
Patient characteristics and comparison between subgroups
REFERENCES
1. Adhiyaman V, Asghar M, Ganeshram KN, Bhowmick BK. Chronic subdural haematoma in the elderly. Postgrad Med J 2002;78:71-5.



2. Balser D, Rodgers SD, Johnson B, Shi C, Tabak E, Samadani U. Evolving management of symptomatic chronic subdural hematoma: experience of a single institution and review of the literature. Neurol Res 2013;35:233-42.



3. Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol 1997;48:220-5.


4. Hamou H, Alzaiyani M, Pjontek R, et al. Risk factors of recurrence in chronic subdural hematoma and a proposed extended classification of internal architecture as a predictor of recurrence. Neurosurg Rev 2022;45:2777-86.



5. Kim SU, Lee DH, Kim YI, Yang SH, Sung JH, Cho CB. Predictive factors for recurrence after burr-hole craniostomy of chronic subdural hematoma. J Korean Neurosurg Soc 2017;60:701-9.



6. Oh HJ, Lee KS, Shim JJ, Yoon SM, Yun IG, Bae HG. Postoperative course and recurrence of chronic subdural hematoma. J Korean Neurosurg Soc 2010;48:518-23.



7. Cofano F, Pesce A, Vercelli G, et al. Risk of recurrence of chronic subdural hematomas after surgery: a multicenter observational cohort study. Front Neurol 2020;11:560269.



8. Kuroki T, Katsume M, Harada N, Yamazaki T, Aoki K, Takasu N. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir (Wien) 2001;143:1041-4.


9. Kolias AG, Chari A, Santarius T, Hutchinson PJ. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol 2014;10:570-8.


10. Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg 2020;141:339-45.


11. Jiang R, Zhao S, Wang R, et al. Safety and efficacy of atorvastatin for chronic subdural hematoma in Chinese patients: a randomized clinicaltrial. JAMA Neurol 2018;75:1338-46.



12. Bartek J Jr, Sjåvik K, Schaible S, et al. The role of angiotensin-converting enzyme inhibitors in patients with chronic subdural hematoma: a Scandinavian population-based multicenter study. World Neurosurg 2018;113:e555-60.


13. Tamura R, Sato M, Yoshida K, Toda M. History and current progress of chronic subdural hematoma. J Neurol Sci 2021 Oct 15;429:118066.

14. Thotakura AK, Marabathina NR. The role of medical treatment in chronic subdural hematoma. Asian J Neurosurg 2018;13:976-83.



15. Ridwan S, Bohrer AM, Grote A, Simon M. Surgical treatment of chronic subdural hematoma: predicting recurrence and cure. World Neurosurg 2019;128:e1010-23.


-
METRICS

-
- 0 Crossref
- 0 Scopus
- 613 View
- 9 Download
- ORCID iDs
-
Chan Yang Noh

https://orcid.org/0000-0002-2489-506XIk Seong Park

https://orcid.org/0000-0002-8187-7204 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print