Introduction
Meticulous watertight dural closure is important after surgery for intradural spinal pathology [
1]. Despite meticulous surgical techniques, a complete watertight closure is impossible, and surgical adhesive (dural sealant) is sometimes applied to reduce the incidence of cerebrospinal fluid (CSF) leakage. The rate of postoperative CSF leakage following spinal surgery with intradural exploration was reported as 5% [
1]. Postoperative CSF leakage can be self-limiting, but in some cases, it leads to complications such as surgical site infection and intracranial hypotension [
2]. Therefore, various surgical adhesives, including dural sealants, have been used to reduce CSF leakage from the primary dura repair site [
3]. BioGlue (CryoLife, Atlanta, GA, USA) is a dural sealant consisting of bovine serum albumin and glutaraldehyde (BSAG) [
3-
6]. Initially, BioGlue was used in cardiovascular surgery to achieve hemostasis and provide mechanical sealing effects, and its usage was expanded into neurological surgeries to augment dura tissue adherence and strength, particularly in reconstructions of the sellar floor after transsphenoidal adenohypophysectomy [
1-
4,
6-
9]. However, BioGlue can gradually self-expand by absorbing surrounding fluid, and it might have a mass effect in a confined space [
4,
5,
8]. Despite dural sealing efficacy, symptomatic neural compression attributable to the expansion of BioGlue was reported as a complication after spine surgery [
4,
5,
8]. We experienced a case of delayed thoracic spinal cord compression caused by expanded BioGlue after the resection of thoracic spinal meningioma. In this case report, we described the painful case to support the proper use of BioGlue and suggested considerable points for its use with a literature review.
Case Report
An 82-year-old-woman visited a hospital in December 2018 for right leg weakness. Her medical history included osteoporosis and dyslipidemia. Neurologic examination revealed hypoesthesia below the T10 dermatome, right leg weakness (manual motor grade IV/V), and increased deep tendon reflex in the lower extremities. Thoracolumbar magnetic resonance imaging (MRI) revealed an intradural extramedullary mass compressing the spinal cord at the T3-4 level with low signal intensity on T2-weighted imaging (
Fig. 1) and the dural tail sign on contrast-enhanced T1-weighted sagittal MRI (
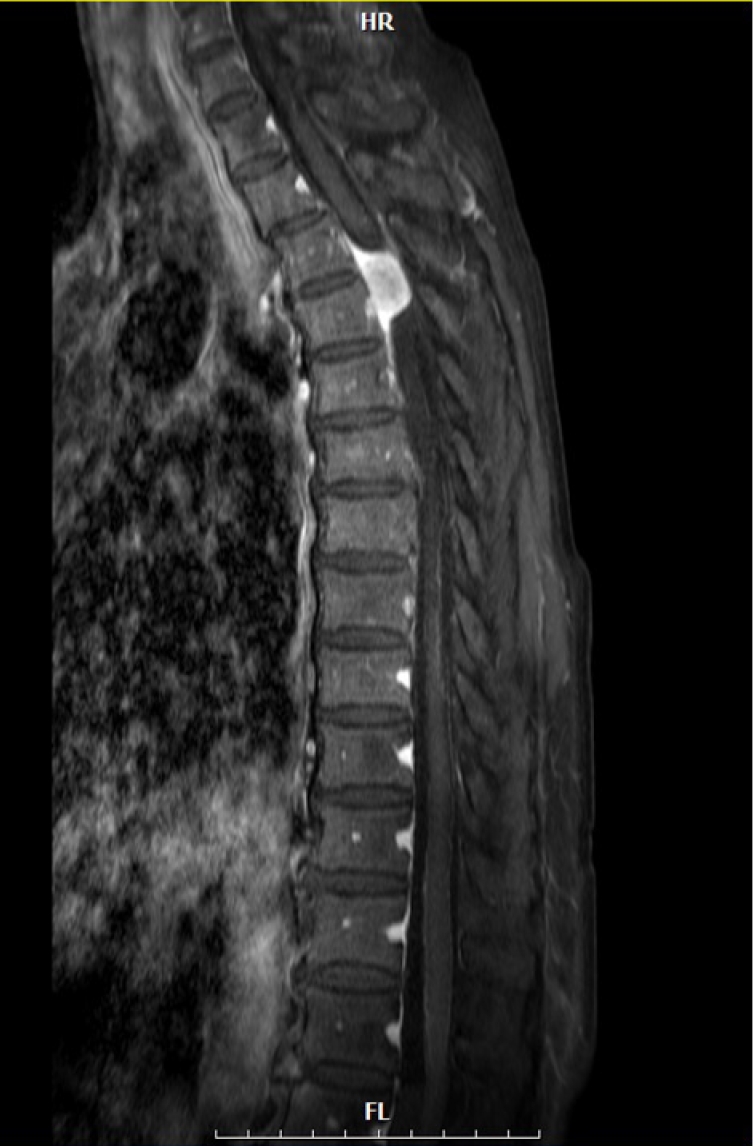
Fig. 2). She underwent T2-3 laminectomy, and tumor resection was performed in December 2018. During surgery, the tumor was completely removed, and the tumor origin was coagulated (Simpson grade II removal) without resection of the dura. The dura mater was primarily closed with 6-0 Prolene in a watertight manner, and BioGlue was applied on the closed dura to augment dural closure. After surgery, her weakness displayed improvement, and leakage of CSF or swelling of the surgical wound did not occur. She was discharged without acute complications on postoperative day 17. Two months after surgery, she visited an outpatient clinic with weakness in both legs (motor grade III/III), which acutely occurred 3 days before presentation. Thoracic spine MRI revealed an epidural mass. The mass exhibited low signal intensity on T2-weighted image, and it compressed the thoracic spinal cord at the previous surgical site (
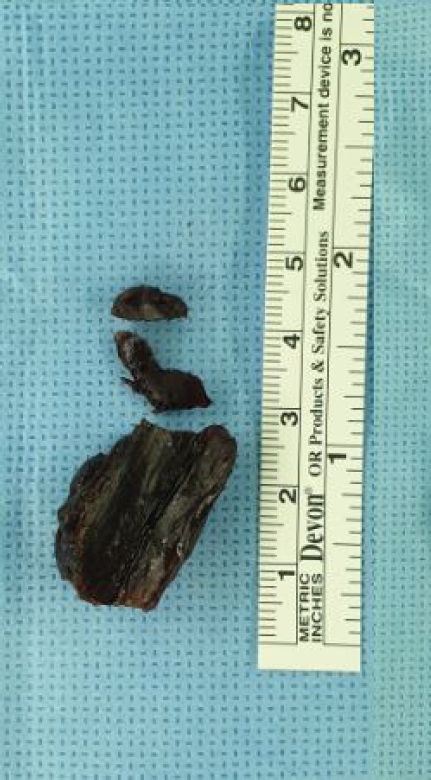
Fig. 3). We performed emergency surgery and removed the epidural mass. During surgery, a hard black-colored mass compressing the spinal dura was completely removed (
Fig. 4). CSF did not leak through the dural closure site, and BioGlue was not applied. Pathological review revealed an inorganic material, which was considered swollen BioGlue. After surgery, the patient’s leg weakness immediately improved to motor grade IV. She was transferred to the Rehabilitation Department with no acute surgical complications. Eighteen months after the second surgery, she could walk independently, but she complained of paresthesia in both legs, which was controlled with medication.
Discussion
We experienced an uncommon but neurologically critical complication after using BioGlue dural sealant for surgery to resect spinal meningioma. Postoperative leakage of CSF is one complication after surgery for spinal intradural pathology [
1-
4,
7-
10]. The reported incidence of CSF leakage requiring intervention after spinal tumor surgery is reported to be as high as 28.6%, and recent studies illustrated that the overall incidence after surgery for both intradural and extradural pathologies is 6.6%±5.8% [
2]. This incidence is significant, and pre-emptive measures might be necessary to augment dural closure. When the dura is opened to address intradural pathology, primary dural closure with sutures was usually performed, and dural sealant was utilized at the surgeon’s discretion [
3,
11].
Among the available sealants, we used BioGlue. BioGlue is a surgical adhesive consisting of BSAG [
3-
6]. After mixing, BioGlue begins to polymerize within 20 to 30 seconds and reaches its bonding strength within 2 minutes. Its sufficient binding strength and biocompatibility have been proven in cardiovascular surgery [
4,
6] as well as brain and spine surgery [
1-
4,
7-
10]. However, its use requires careful application. Stylli et al. [
10] demonstrated that BioGlue applied directly to the exposed surface of the normal brain induces a minimal inflammatory response, and activated glial cells expressing glial fibrillary acidic proteins underwent in rats [
5]. BioGlue elicited a long-term inflammatory response characterized by active inflammation surrounding the glue remnant together with many granulocytes, histiocytes, and multinucleated giant cells [
10,
12-
14]. The chronic inflammation inducted by BioGlue resulted in late complications such as wound infection or mass effects [
4,
5,
14]. Therefore, BioGlue can induce neurological complications if it is applied in a thick layer [
8,
15,
16].
In this case, thoracic myelopathy caused by the tumor was improved after surgery, and the patient was discharged days after surgery with symptom improvement. However, delayed neurological deterioration occurred 2 months after surgery. The epidural mass was characterized by dark signal intensity on T2-weighted images at the previous operation site (T3-4 level). The MRI findings of the epidural mass correspond to previously reported data [
17]. Emergency removal of the hard BioGlue mass alleviated spinal cord compression and improved paraparesis, but dysesthesia remained. Strong suspicion of potential complications after the use of BioGlue led to a prompt decision for the patient, but a care surgical technique is required when using BioGlue. Many spinal surgeons apply BioGlue in a thin layer (1-2 mm) to ensure dural closure and prevent mass effects induced by delayed swelling of BioGlue [
4,
18]. Although we did not measure the thickness of BioGlue, it exceeded the recommended thickness, and the thick BioGlue layer induced delayed thoracic myelopathy and neurological sequelae. Although the use of dural sealant might be advantageous in reducing complications associated with CSF leakage, it should be applied in a thin layer to achieve an optimal outcome without iatrogenic complications.
In addition to careful application, strong suspicion of potential complications was important for prompt decision-making. The patient presented with a neurological deficit, and the primary differential diagnosis included cerebral stroke. The medical team should be aware of the potential complications of BioGlue to ensure that they are included in the differential diagnosis. However, the interval from initial application to the detection of a mass effect induced by BioGlue has varied from several days to 2 years [
4,
5,
18]. Yuen and Kaye reported that unlike other sealants, which degraded within 4 to 9 weeks, BioGlue can remain in place until 2 years after spinal surgery [
9]. The product information of BioGlue does not specify the time to biodegradation. Although BioGlue created a long-lasting hard barrier to seal the closed dura, it might not be suitable for use in a confined surgical field such as spinal laminectomy sites because of its prolonged persistence. It may be suitable for use after transsphenoidal surgery, but its use in spine surgery could require caution.
Conclusion
We reported a case of delayed complications after using BioGlue in spine tumor surgery. Although the complication arose 2 months after surgery, suspicion of the potential complication permitted a prompt surgical decision. Although BioGlue is an effective dural sealant, surgeons should be aware of its long-lasting mass effects, which are detrimental to neural structure. We should understand the characteristics of various dural sealants to optimize patient outcomes. If a BioGlue is selected, it should be applied in a thin layer.













 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print