|
 |
| J Korean Soc Geriatr Neurosurg > Volume 19(2); 2023 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
Fig. 1.
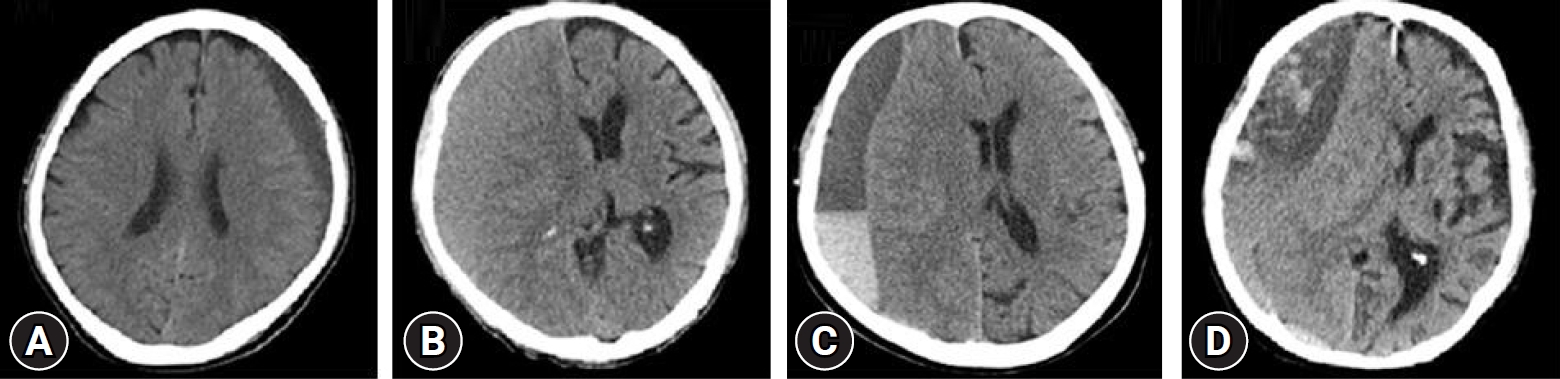
Table 1.
Table 2.
Table 3.
Table 4.
|
Chronic alcoholism(+) |
P-value |
Head trauma(+) |
P-value | |||
|---|---|---|---|---|---|---|
| Operation(-) | Operation(+) | Operation(-) | Operation(+) | |||
| Antiplatelet | 3 | 20 | 0.018 | 10 | 24 | 0.043 |
| Anticoagulant | 1 | 6 | 2 | 6 | ||
| None | 11 | 11 | 53 | 50 | ||
REFERENCES
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 739 View
- 11 Download
- ORCID iDs
-
Je Han Hwang

https://orcid.org/0009-0004-6958-0530Min Ho Kong

https://orcid.org/0000-0001-8543-4147Jung Hee Kim

https://orcid.org/0000-0001-5914-2482Se Yeon Jang

https://orcid.org/0000-0003-4337-5972Sung Hoon Kim

https://orcid.org/0000-0001-5557-5897Soono Hong

https://orcid.org/0000-0003-2471-2777Tae Gon Kim

https://orcid.org/0000-0001-6258-6412 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print