|
 |
| J Korean Soc Geriatr Neurosurg > Volume 19(2); 2023 > Article |
|
Abstract
Objective
This nationwide longitudinal follow-up study investigated the relationship between ischemic stroke and ossification of the posterior longitudinal ligament (OPLL).
Methods
Patient data were gathered from the National Health Insurance Service Health Screening cohort. Patients with OPLL were identified by the International Classification of Diseases, 10th revision codes M48.8, M48.81, M48.82, and M48.83. Age- and sex- stratified matching at a 1:5 ratio was used to include a total of 1,289 patients with OPLL and 6,445 controls from January 1, 2004 to July 31, 2015. The Kaplan-Meier method was used to calculate the ischemic stroke incidence rate for each group. Cox proportional-hazards regression analysis was used to estimate the hazard ratio of ischemic stroke.
Results
Ischemic stroke occurred in 92 (7.1%) patients in the OPLL group and 162 (2.5%) patients in the control group had ischemic stroke. Adjusting for age and sex, the hazard ratio of ischemic stroke in the OPLL group was 4.692 (95% confidence interval [CI], 3.610-6.099). The hazard ratio of ischemic stroke in the OPLL group was 5.008 (95% CI, 3.845-6.516) when considering additional income and underlying diseases. The subgroup analysis showed higher risk ratios of ischemic stroke in all OPLL patient subgroups defined in terms of sex, age, diabetes, hypertension, and dyslipidemia.
The ossification of posterior longitudinal ligament (OPLL) is a multifactorial disease made by abnormal bone formation that serves to replace spinal ligamentous tissue [1-5]. There are no established facts about the exact cause or mechanism of OPLL [6]. When OPLL develops, it narrows the spinal canal, causing damage to the spinal cord. As a result, patients show clinical symptoms of myelopathy that seriously deteriorate the quality of life [7].
Until now, no studies have investigated the direct connection between ischemic stroke and OPLL. However, ischemic stroke which is a serious disease with functional impairment is related with factors such as infection and atherosclerosis. Through our nationwide longitudinal study, we tried to reveal the association between ischemic stroke and OPLL. We also tried to clarify OPLL increases the incidence of ischemic stroke.
In South Korea, there is a form of universal health insurance that covers essential medical expenses for all residents under a single public scheme, National Health Insurance Service (NHIS). NHIS provides a general health examination for full time and temporary workers above the age of 40 every year or every two years. It also collects individual data including medical examination with demographic characteristics in the National Health Information Database (NHID). For our study, we have lawfully obtained the right to access the NHID health examination data from 2004 to 2015 with help of the Institutional Review Boards (IRB No. 2020-01-011) of the CHA Bundang Medical Center [8].
The total number of 514,557 subjects subjects underwent national health examination and were followed for 12 years until December 31, 2015. The patients with International Classification of Diseases 10th Revision (ICD-10) codes, ‘M48.8, M48.80, M48.81, M48.82, M48.83’, were selected as the OPLL patients (n=3,405). Among those patients, 1,977 subjects are excluded due to lack of computed tomography data. Ones newly diagnosed with OPLL after January 1, 2003 in the remaining group of 1,428 subjects were selected as primary cohorts for our study, which amounted to a total of 1,289. With the ‘Match IT’ R package algorithm, 6,445 subjects were enrolled for the controls through 1:5 age- and sex- stratified matching (Fig. 1). The subjects of the study were followed from the initial date of ischemic stroke diagnosis until their death or the end of the observation period. The occurrence of ischemic stroke was analyzed after controlling age, sex, income, and underlying diseases.
Both chi-square test and Student’s t-test were utilized to contrast the demographic factors between the OPLL group and the control group. The Kaplan-Meier method was used to estimate the Ischemic Stroke-free survival probability in each group. Multivariate analysis of the Cox proportional-hazards regression model was used for searching the impact of OPLL on the occurrence of the ischemic stroke. In detail, we made two corrected models, in which model 1 was adjusted for age and sex and model 2 was adjusted for age, sex, income, and medical factors including hypertension, diabetes, and dyslipidemia as covariates. The Cox proportional-hazards regression model and R software version 3.3.3 were used for subgroup analyzes to adjust covariates.
For the newly diagnosed OPLL group and control group, more part of the subjects were female (51.7%) and an average age was 57.4±9.65 (years). There were some factors which showed significant difference between two groups. One is the diabetes mellitus which has a higher prevalence in the control group (13.0%) than in the experimental group (9.9%; P=0.002). In terms of hypertension, the control group (41.8%) had a higher prevalence than the other group (36.5; P<0.001). In respect of the dyslipidemia a higher incidence was observed in the control group (17.4%) than in the OPLL group (14.5%; P=0.013) (Table 1).
In terms of ischemic stroke, the experimental group (10.687) has a higher incidence rate than the control group (2.011), expressing 1,000 person-year as a unit. Also we found out that its hazard ratio was 4.692 (95% confidence interval [CI], 3.610-6.099) compared to the control group in model 1. In model 2, it was 5.008 (95% CI, 3.845-6.516) (Table 2). In the Fig. 2, when follow-up for ischemic stroke was carried out for 12 years, the cumulative incidence of the experimental group showed a higher number than that of the control group.
There are ten subgroups in respect to gender, age, diabetes mellitus, hypertension, and dyslipidemia (Table 3). For all subgroup factors, the incidence rate of ischemic stroke was different between OPLL group and control group. Regarding the incidence of ischemic stroke, there was difference in both male (95% CI, 2.948-6.174) and female (95% CI, 3.724-7.891). There was also significantly deference in age <65 group (95% CI, 4.219-9.111), age ≥65 group (95% CI, 2.538-5.227), non-diabetes mellitus (95% CI, 3.722-6.627), diabetes mellitus (95% CI, 2.376-8.409), non-hypertension (95% CI, 3.591-7.888), and hypertension (95% CI, 3.298-6.694). Lastly, significantly different incidence of ischemic stroke could be observed in both non-dyslipidemia (95% CI, 3.682-6.643) and dyslipidemia (95% CI, 2.425-7.769).
Our countrywide longitudinal research found that the OPLL group had 4.692 fold greater incidence of ischemic stroke after controlling sex and age. The OPLL group also had 5.008 fold greater incidence of ischemic stroke after controlling sex, age, wealth, hypertension, dyslipidemia, and diabetes mellitus. In addition, our study showed that the incidence of ischemic stroke was greater in the OPLL group than in the control group in sex, age, non-diabetes, diabetes, non-hypertension, hypertension, non-dyslipidemia, and dyslipidemia subgroups.
Until now, no studies have examined the direct connection between OPLL and ischemic stroke. Instead, several papers have shown that cytokines and inflammation are associated with ischemic stroke. Toma and McCaffrey [9] reported transforming growth factor-β (TGF-β) plays a major role in creating atherosclerosis of blood vessel. It prompts the chemotaxis of repair tissue cells, regulates immunity, and leads to matrix proliferation. Also it negatively regulates antiproliferative processing and apoptotic effects [10-12]. The atherosclerosis of blood vessel can make a thrombosis or block vessels, which leads to ischemic stroke [13-15]. In the development of OPLL, several genes such as TGF-β and bone morphogenetic protein (BMP) are involved. TGF-β/BMP signaling regulates the processing necessary for osteoblastogenesis that promotes progression of OPLL [5]. Thus, this cytokine TGF-β could explain a connection between OPLL and ischemic stroke.
Secondly, Lindsberg and Grau [16] reported systemic markers of inflammation such as the high leukocyte count, fibrinogen, and C-reactive protein (CRP) have been shown to be risk markers of the ischemic stroke. This is because these markers serve as an indication of acute or chronic infectious status. In the infectious tissue, many inflammatory cells secrete inflammatory cytokines into the blood that promote the formation of atherosclerosis and atherosclerotic plaque [4,16-21], which creates thrombosis and increases the risk of ischemic stroke. In addition, Kawaguchi et al. [22] reported a statistical difference in the CRP concentration between the OPLL patients and the non-OPLL patients. The mean serum CRP concentration was 0.122±0.141 mg/dL in the OPLL group and 0.086±0.114 mg/dL in the other group (P=0.047). This means that the CRP concentration was significantly greater in the OPLL patients, so acute or chronic infectious state can indicate a relationship between OPLL and ischemic stroke.
The limitations of our longitudinal cohort study are as follows. First, although our analysis adjusted for sex, age, income, hypertension, diabetes, and dyslipidemia aspects of subjects, our study did not consider the impact of patients’ lifestyle, including the degree of smoking, drinking, exercising, and obesity status. These lifestyle factors may have a crucial impact on the occurrence of ischemic stroke. Secondly, the NHIS database did not have enough information on biomarkers that are related to inflammatory. As a result, it is difficult to understand the exact relationship between OPLL and ischemic stroke through inflammatory markers. Nevertheless, this is the first study attempting to find the connection between ischemic stroke and OPLL in Korean patients. Also it is the largest nationwide longitudinal study indicating an increased risk of ischemic stroke in OPLL patients.
Acknowledgments
This work was supported by a grant of Basic Science research Program through the NRF funded by the Ministry of Science, ICT and future Planning (RS-2023-00209591) and by Ministry of Education (RS-2023-00243616).
Fig. 1.
Flowchart of cohort establishment. This research was a 12-year-long study from the NHIS-HEALS cohort. OPLL, ossification of the posterior longitudinal limit; ICD-10, International Classification of Diseases, 10th revision; CT, computed tomography.

Fig. 2.
Kaplan-Meier curves with cumulative hazards of ischemic stroke in the ossification of the posterior longitudinal ligament (OPLL) group and the control group.
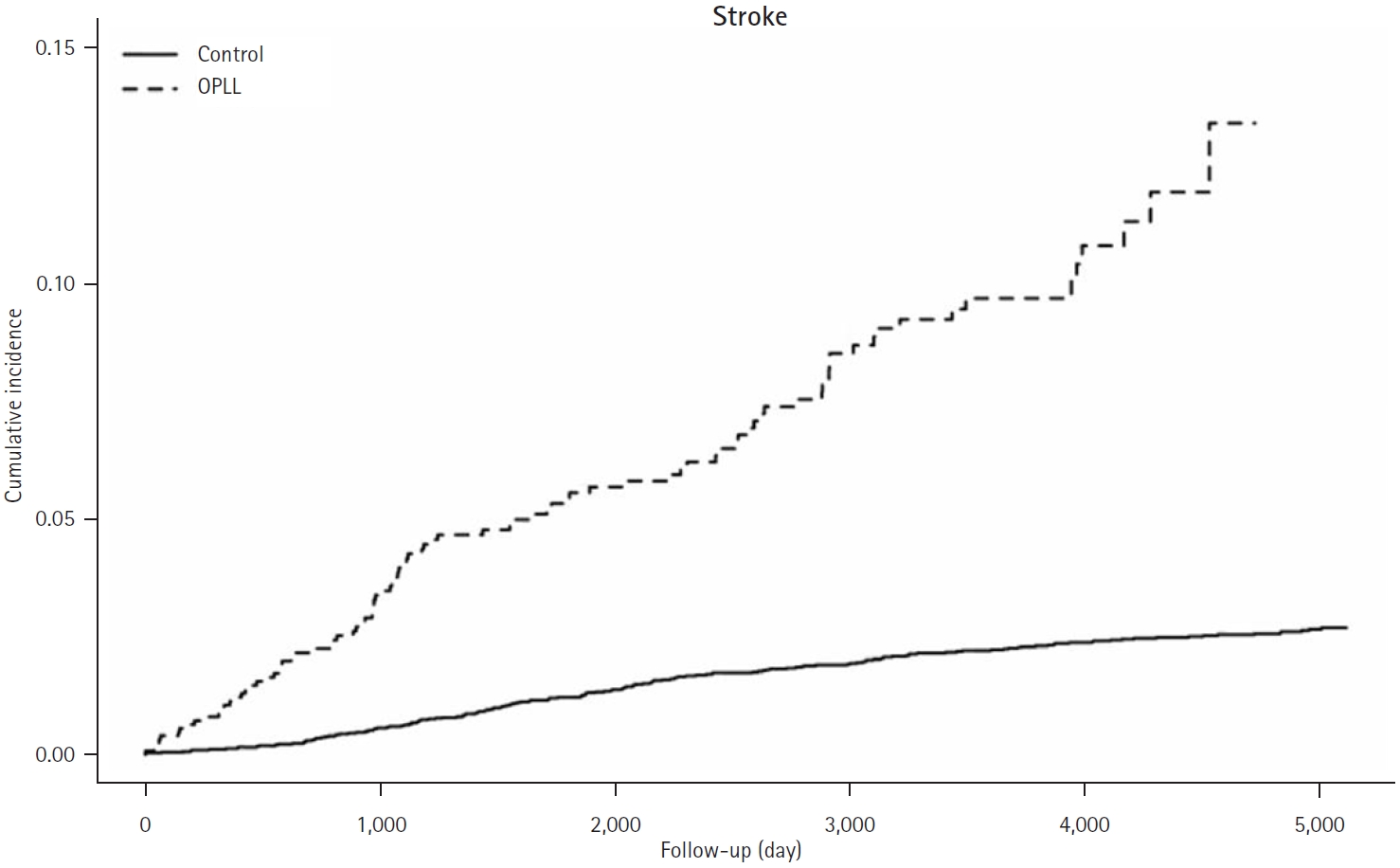
Table 1.
Characteristics of patients in the OPLL and control groups
Table 2.
Adjusted hazard ratios for ischemic stroke in the OPLL and control groups
Table 3.
The incidence rate of ischemic stroke in subgroup analyses between the OPLL and control groups
REFERENCES
1. Oshima Y, Doi T, Kato S, et al. Association between ossification of the longitudinal ligament of the cervical spine and arteriosclerosis in the carotid artery. Sci Rep 2020;10:3369.



2. Saito H, Yayama T, Mori K, et al. Increased cellular expression of interleukin-6 in patients with ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2023;48:E78-86.


3. Sakou T, Matsunaga S, Koga H. Recent progress in the study of pathogenesis of ossification of the posterior longitudinal ligament. J Orthop Sci 2000;5:310-5.


4. Tung NT, He Z, Makino H, et al. Association of inflammation, ectopic bone formation, and sacroiliac joint variation in ossification of the posterior longitudinal ligament. J Clin Med 2023;12:349.



5. Yan L, Gao R, Liu Y, He B, Lv S, Hao D. The pathogenesis of ossification of the posterior longitudinal ligament. Aging Dis 2017;8:570-82.



6. Won YI, Lee CH, Yuh WT, Kwon SW, Kim CH, Chung CK. Genetic odyssey to ossification of the posterior longitudinal ligament in the cervical spine: a systematic review. Neurospine 2022;19:299-306.



7. Hou X, Sun C, Liu X, et al. Clinical features of thoracic spinal stenosis-associated myelopathy: a retrospective analysis of 427 cases. Clin Spine Surg 2016;29:86-9.


8. Lee DH, Sheen SH, Lee DG, et al. Association between ischemic stroke and seropositive rheumatoid arthritis in Korea: A nationwide longitudinal cohort study. PLoS One 2021;16:e0251851.



9. Toma I, McCaffrey TA. Transforming growth factor-β and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res 2012;347:155-75.



10. Singh NN, Ramji DP. The role of transforming growth factor-beta in atherosclerosis. Cytokine Growth Factor Rev 2006;17:487-99.


11. Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke 1996;27:852-7.


12. Harvey BK, Hoffer BJ, Wang Y. Stroke and TGF-beta proteins: glial cell line-derived neurotrophic factor and bone morphogenetic protein. Pharmacol Ther 2005;105:113-25.


13. Handa N, Matsumoto M, Maeda H, Hougaku H, Kamada T. Ischemic stroke events and carotid atherosclerosis: results of the Osaka Follow-up Study for Ultrasonographic Assessment of Carotid Atherosclerosis (the OSACA Study). Stroke 1995;26:1781-6.


14. Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med 1994;331:1474-9.


15. Kim J, Cha MJ, Lee DH, et al. The association between cerebral atherosclerosis and arterial stiffness in acute ischemic stroke. Atherosclerosis 2011;219:887-91.


16. Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke 2003;34:2518-32.


17. Mauriello A, Sangiorgi G, Fratoni S, et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol 2005;45:1585-93.


18. Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke 2012;7:378-85.



19. McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience 2009;158:1049-61.


20. Lee SH, Kim H, Han IB, Sheen SH, Hong JB, Sohn S. Association between ischemic stroke and pyogenic spondylitis in Korea: nationwide longitudinal cohort study. J Cerebrovasc Endovasc Neurosurg 2023;25:143-9.



-
METRICS

-
- 0 Crossref
- 0 Scopus
- 707 View
- 16 Download
- ORCID iDs
-
Seil Sohn

https://orcid.org/0000-0001-5724-8099 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print