Outcomes of chronic subdural hematoma and their correlations with old age
Article information
Abstract
Objective
This study explored the prognostic factors of chronic subdural hematoma in patients who underwent burr hole drainage, both overall and in elderly patients.
Methods
This study enrolled 120 patients with chronic subdural hematoma who underwent burr hole drainage at a single center between January 2016 and December 2020. Old age was defined as ≥65 years, and a good prognosis was defined as a decrease in the modified Rankin scale from admission to the last follow-up. Factors correlated with good prognosis in elderly patients compared with total patients were evaluated using the chi-square test and logistic regression analysis.
Results
Among the 120 patients, 66 (55.0%) were ≥65 years old, and 76 (63.3%) had severe symptoms on admission. Old age (≥65) was the only factor significantly correlated with severe symptoms (P=0.021; odds ratio [OR], 2.618; 95% confidence interval [CI], 1.154–5.942). The following variables were significantly associated with the prognosis in both groups: diabetes (total: P=0.008; OR, 0.207; 95% CI, 0.064–0.664; old age: P=0.011; OR, 0.172; 95% CI, 0.044–0.672) and severe symptoms on admission (total: P<0.001; OR, 6.994; 95% CI, 2.771–17.399; old age: P=0.007; OR, 6.177; 95% CI, 1.656–23.046).
Conclusion
No difference in prognostic factors was observed between the overall patients and elderly patients with chronic subdural hematoma who underwent burr hole drainage. In both groups, patients without diabetes or with severe symptoms during hospitalization showed better recovery after burr hole drainage.
Introduction
Chronic subdural hematoma (CSDH) is one of the common types of intracranial hemorrhage, especially more frequently in elderly people [1,2]. If patients have symptoms caused by the mass effect of CSDH, burr hole drainage, a simple and effective operation, is recommended [3,4]. Risk factors of CSDH, such as minor head trauma, coagulopathy, and chronic alcohol use, are previously well known, and among them, old age is a strong risk factor due to brain atrophy [5–7]. Furthermore, prognostic factors are known to be similar to the risk factor in many studies but they remain controversial [8–10]. Therefore, this study aimed to explore the prognostic factors of CSDH in patients who underwent burr hole drainage and compare them with old age.
Material and Method
Study design
Between January 2016 and December 2020, 138 patients were admitted and treated with CSDH in a single center. Inclusion criteria were patients who underwent burr hole drainage due to the mass effects of CSDH and had a follow-up duration of >3 months postoperatively. A total of 15 patients who were lost to follow-up and 3 patients without burr hole drainage were excluded. Finally, a total of total 120 patients were enrolled. This study was approved by the institutional review board of Ulsan University Hospital, and informed consent was waved (UUH 2021-08-007).
Patient information was obtained with the retrospective chart review, including gender, age, diabetes, hypertension, end-stage renal disease (ESRD), stroke, heart disease, and alcoholism history; anticoagulant and antiplatelet agents; and head injury. Old age was defined as >65 years; diabetes as taking insulin and/or oral hypoglycemic agents; hypertension as controlling blood pressure with oral hypertensive agents; ESRD as kidney failure (glomerular filtration rate of <15 mL/min/1.73 m2) needed permanent renal replacement therapy; stroke as intracranial hemorrhage due to cerebral vessel problem and/or cerebral infarction; heart disease as cardiomyopathy, coronary artery disease, and/or myocardial infarction; alcoholism as drinking alcohol exceeding 14 standard drinks per week or 4 drinks per day; and head trauma as the presence of head injury 3 months before hospital admission [11].
Burr hole drainage
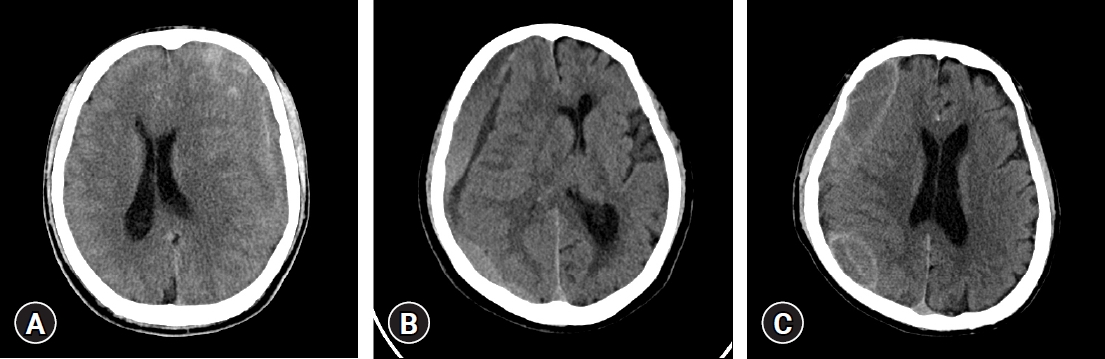
Patients who complain of symptoms, such as headache, general weakness, and/or neurologic deficit, were diagnosed with CSDH through brain computer tomography (CT) scan. If CSDH was observed in brain CT scan, checking hematoma thickness; midline shifting; hematoma density classified as high, iso, low, and mixed based on brain parenchymal density; and hematoma location as unilateral or bilateral was performed (Fig. 1) [9]. The mixed type included multilayered, separated, and trabecular types (Fig. 2) [12]. In addition, patient status was examined and classified based on the modified Rankin scale (mRS). Severe symptoms on admission were defined as mRS score of ≥2. According to the operator’s discretion about the mass effect of hematoma, a single burr hole was trephined at the hematoma site with the maximal diameter and warm physiological saline irrigated until draining clear saline was under general anesthesia. After inserting a silicone tube in the subdural space, the drainage was closed after 1 to 3 days. If it was sufficiently removed at the postoperative brain CT scan and/or symptoms were improved, the silicone tube was removed.
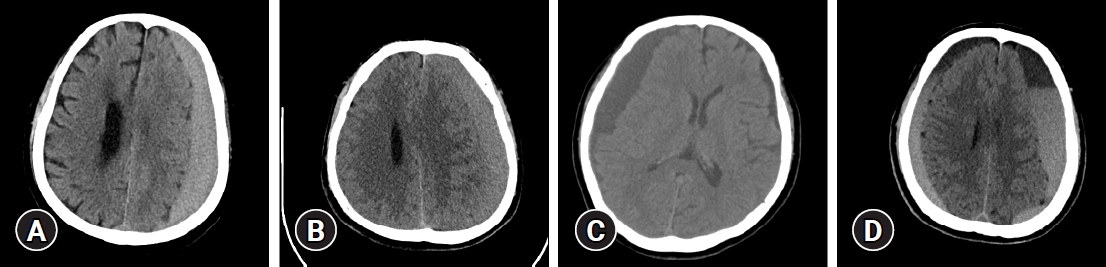
Hematoma density was classified as high, iso, low, and mixed based on brain parenchymal density on brain computed tomography. (A) High, (B) iso, (C) low, (D) mixed.
Follow-up
After hospital discharge, brain CT scans were examined at 1 month and 3 months postoperatively through the outpatient department. Recurrence was defined as increasing hematoma thickness based on a brain CT scan at 3 days postoperatively and/or aggravating symptoms. If necessary, burr hole drainage was performed again. As in admission, patient status classified as mRS was evaluated at the last follow-up, and good recovery was defined as decreasing mRS score from admission to the last follow-up.
Statistics
Statistics analyses were performed with the IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). Baseline characteristics were performed chi-square test, Fisher exact test, or Student t-test to compare the old age and other groups. Subgroup analysis was performed to investigate the values correlated with severe symptoms on admission. The chi-square test, Fisher exact test, or Student’s t-test were performed on the clinical, radiologic, and neurologic data. Furthermore, logistic regression analysis was performed to confirm the factors related to good recovery in the total and old age groups. In all analyses, P≤0.05 was defined as statistically significant.
Results
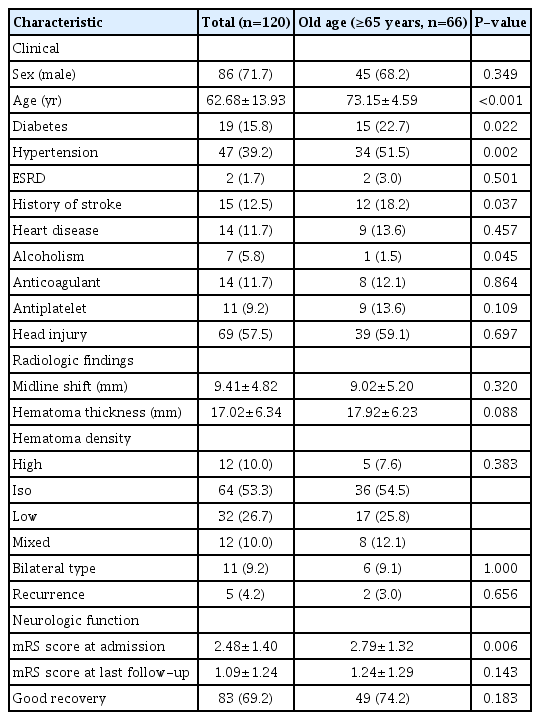
Among 120 patients who underwent burr hole drainage, 66 (55.0%) were classified into the old age group. The mean ages of the old age and total groups were 73.15±4.59 (years) and 62.68±13.93 (years), respectively. In baseline characteristics, several variables in the old age group, such as diabetes (P=0.022), hypertension (P=0.002), history of stroke (P=0.037), and mRS score on admission (P=0.006), were significantly higher than the other group, whereas only alcoholism (P=0.045) was lower (Table 1).
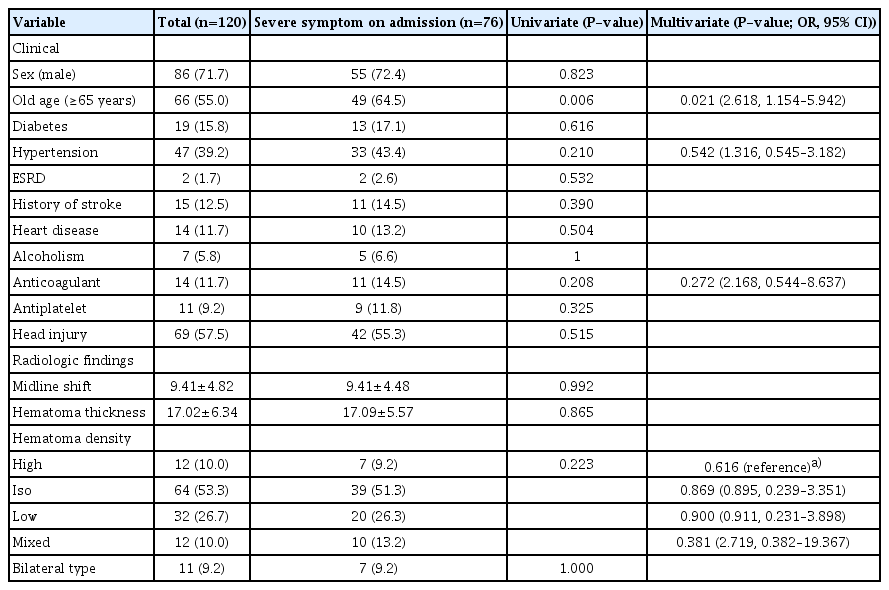
A total of 76 (63.3%) patients had severe symptoms on admission, and only old age was correlated with severe symptom significantly in both univariate (P=0.006) and multivariate analyses (P=0.021, odds ratio [OR], 2.618; 95% confidence interval [CI], 1.154–5.942) (Table 2).
In variables associated with good recovery, 86 (71.7%) patients in the total group and 49 (74.2%) patients in the old age group had a good prognosis. In the total group, diabetes (univariate P=0.025; multivariate P=0.008, OR, 0.207; 95% CI, 0.064–0.664) and severe symptoms on admission (univariate P<0.001; multivariate P<0.001, OR, 6.994; 95% CI, 2.771–17.399) were statistically significant in both univariate and multivariate analyses. In the old age group, diabetes (P=0.015), hematoma thickness (P=0.043), and severe symptoms on admission (P=0.008) were significantly in univariate analysis, whereas diabetes (P=0.011, OR, 0.172; 95% CI, 0.044–0.672) and severe symptoms on admission (P=0.007, OR, 6.177; 95% CI, 1.656–23.046), except hematoma thickness (P=0.082, OR, 1.012; 95% CI, 0.916–1.117), were significant in multivariate analysis. Recurrence was only observed in 4 patients in the total group and 2 in the old age group, and no correlation with prognosis was observed in univariate analysis (P=1.000) (Table 3).
Discussion
CSDH is known as an intracranial hemorrhage relatively simple surgery and good prognosis [13,14]. However, among the patients with CSDH, poor symptoms and/or bad outcomes were observed. To prevent poor outcomes, some studies evaluated and identified prognostic factors, such as coagulating factors, postoperative pneumocephalus, and alcoholism [8,10]. Especially among them, old age was known as a significant factor due to low compliance caused by brain atrophy but remains controversial [15].
In our study, good recovery is more frequent in both the total (n=86, 71.7%) and old age (n=49, 74.2%) groups. Although no statistically significant difference was observed, the proportion of patients with good recovery was higher in the old age group than in the total group. This result suggests that the rate of severe symptoms on admission was higher in the old age group; thus, the recovery rate was greater. A subgroup analysis was performed to confirm the relationship between severe symptoms on admission and the old age group. Regarding the symptom severity on admission, old age was only the associated factor. It is suggested that no symptom lasts until the limit due to a wider subdural space caused by brain atrophy and symptoms sudden worsening after the breaking point in old age. Therefore, poor outcomes are considered to have a higher rate of severe symptoms on admission than the problem of old age.
Studies on prognostic factors in our study found that patients without diabetes or with severe symptoms on admission are associated with good recovery in both the old age and total groups. Diabetes is a glucose control disorder, thereby causing peripheral blood circulation disorders [16]. Therefore, decreased microcirculation due to diabetes is considered to be a factor that inhibits brain function recovery decreased due to the mass effects. Furthermore, systemic complications caused by diabetes are thought to be a factor that inhibits the recovery of systemic functions. In addition, older age has a higher rate of hypertension and diabetes and a higher rate of stroke history. With this, recovery is expected to be delayed; however, the degree of actual recovery is higher; hence, old age is not considered a factor directly impeding recovery.
Moreover, some studies reported that initial patient status was correlated with outcomes [9,17]. Amirjamshidi et al. [18] reported that a lower initial Glasgow coma scale and higher Glasgow outcome scale at discharge were associated with poor outcomes. Conversely, in our study, severe symptoms on admission were correlated with a good recovery. These different results of the 2 studies were suggested due to different outcome definitions. Most studies judged outcomes according to only the patient’s condition a few months postoperatively. However, in our study, to prevent compounding effects, the initial patient status affects the outcome, and to reflect only the effects of operation and treatment, decreasing mRS score from admission to the last follow-up was considered a good recovery. Therefore, this result is affected by different definitions of good outcomes between the 2 studies. In addition, this result is thought to be caused by the fast recovery after burr hole operation in patients with CSDH and rarely causes permanent sequelae even when severe symptoms occur. Therefore, burr hole can be recommended to patients with CSDH who visited a hospital with severe symptoms to prevent the occurrence of permanent sequelae.
Limitation
This study has several limitations. First, this study is limited by its retrospective nature. Therefore, it could have selective bias and compounding effects. To prevent this problem, other factors related to the procedure were set to the same, and the outcome was set to exclude the initial state. Second, some differences in baseline characteristics were observed between the total and old age (≥65) groups. Despite these differences, diabetes and severe symptoms on admission were statistically significant. Therefore, these factors are considered significant. Third, this is a small-sized single center study. To accurately determine other prognostic factors, well-planned and larger prospective studies performed in a multicenter would be necessary.
Conclusion
No difference was observed in prognostic factors between the overall patient and old age groups in patients with CSDH who underwent burr hole drainage. In both groups, patients without diabetes or with severe symptoms during hospitalization were confirmed to show better recovery after burr hole drainage.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.