Introduction
Schwannomas occur most frequently within the intradural-extramedullary compartment. However, intramedullary schwannoma is a very rare intraspinal cord tumor. It accounts for only 1.1% of spinal schwannoma [
1]. It is a disease that occurs in commonly in middle age [
2]. However, this case has clinical significance in that it occurred in an elderly person.
We report a rare case of thoracic intramedullary schwannomas in elderly explored by magnetic resonance imaging (MRI) and pathogenesis within the spinal cord.
Case Report
A 71-year-old female patient was admitted to our hospital with gradual paraparesis with urinary difficulty for 10 days. She had no previous spinal disease, nor any traumatic history.
Neurological examination revealed that the motor power of the patientŌĆÖs left leg was grade IV and right leg GIII. The sensation of all modalities under T5 dermatomes was reduced. Her bilateral knee jerks and ankle jerks were hyperactive and well sustained ankle clonus was observed. The lesion showed diffuse bulbous thickening with ill-defined central signal change of spinal cord from C6 to T6 level on the T2 weight-image of MRI (
Fig. 1). An ovoid, well-marginated enhanced mass was located at the center of the cord on the gadolinium enhanced image (
Fig. 2).
The operation was performed with standard posterior midline approach. After laminoplasty from T2 to T5, a midline dura incision with lateral retraction was performed. Midline myelotomy showed reddish and some infiltrative adhesive mass lesion. Frozen section of the lesion was defined as a schwannoma. Due to the infiltrative adhesive nature, its total resection was not easy. During the procedure, the intraoperative monitoring was uneventful. After the operation, the patientŌĆÖs weakness was improved, and IV+ and self-voiding was possible. Histological analysis confirmed the diagnosis of schwannoma (
Figs. 3-
5).
Discussion
James Kernohan has been recognized as the first neurosurgeon to report an intramedullary schwannoma case in 1952. However, Penfield had already reported an intramedullary tumor with schwannomas characteristics in 1932 [
3].
Intramedullary schwannoma is a rare disease among non- neurofibroma patients, accounting for 0.3% of intraspinal schwannomas and 1.1% of spinal schwannoma [
4]. The rarity of intramedullary schwannoma is due to the absence of Schwann cells in the central nerve system including the parenchyma of the brain and spinal cord, thus the pathogenesis of intramedullary schwannoma is still controversial. None of specific theories has gained acceptance. There are 5 hypotheses: (1) the intramedullary perivascular nervous plexus along Schwann cells, (2) the migration of Schwann cells during embryonic development, (3) the aberrant intramedullary nerve fibers related to Schwann cells, (4) neoplastic growth of Schwann cells in dorsal root entering zone, and (5) focal intramedullary proliferation of Schwann cells in reaction to chronic diseases or trauma [
5-
7].
The ratio of male to female of intramedullary schwannomas is 3:1. The mean age of disease presentation was 40.2 years, middle age [
2]. The common region of intramedullary schwannomas is the cervical spine (63%), the thoracic spine (26%), and the lumbar (11%) levels [
8].
MRI is one of the choices for the diagnosis of spinal cord tumors. Intramedullary Schwannoma is often known to be hypointense on T1 weighted image, and hyperintense on T2 weighted image [
9]. However, signal intensity depends on macroscopic features and histological composition such as cysts, hemorrhage, and necrosis [
5]. Thus diagnostic MRI of the intramedullary schwannoma is very limited and pathological analysis is important for diagnosis.
Common pathological finding is Antoni type A or mixture with Antoni type B. Schwannoma cell cytoplasm was reactive to S-100 protein but non-reactive to glial fibrillary acidic protein and epithelial membrane antigen [
10].
The main treatment of schwannoma is surgical excision. Complete removal of tumor is usually possible and prognosis of disease is satisfactory [
7,
11].
Conclusion
Despite the rarity of intramedullary schwannoma it should be considered as one of the possible tumors in intramedullary lesion. Although this this disease is common in middle-aged people, it is a disease that should require differential diagnosis even in geriatric patients. The main treatment of the disease is its surgically total excision; however, its adhesive nature makes the removal difficult. It is essential to recognize the adhesive nature of the intramedullary schwannoma.
Acknowledgments
We thank the patient for her valuable participation in this study.
Fig.┬Ā1.
A T2 weighted sagittal image showing diffuse bulbous thickening with ill-defined central signal change of the spinal cord, from C6 to T6. Written informed consent was obtained for publication of this case report and accompanying images.
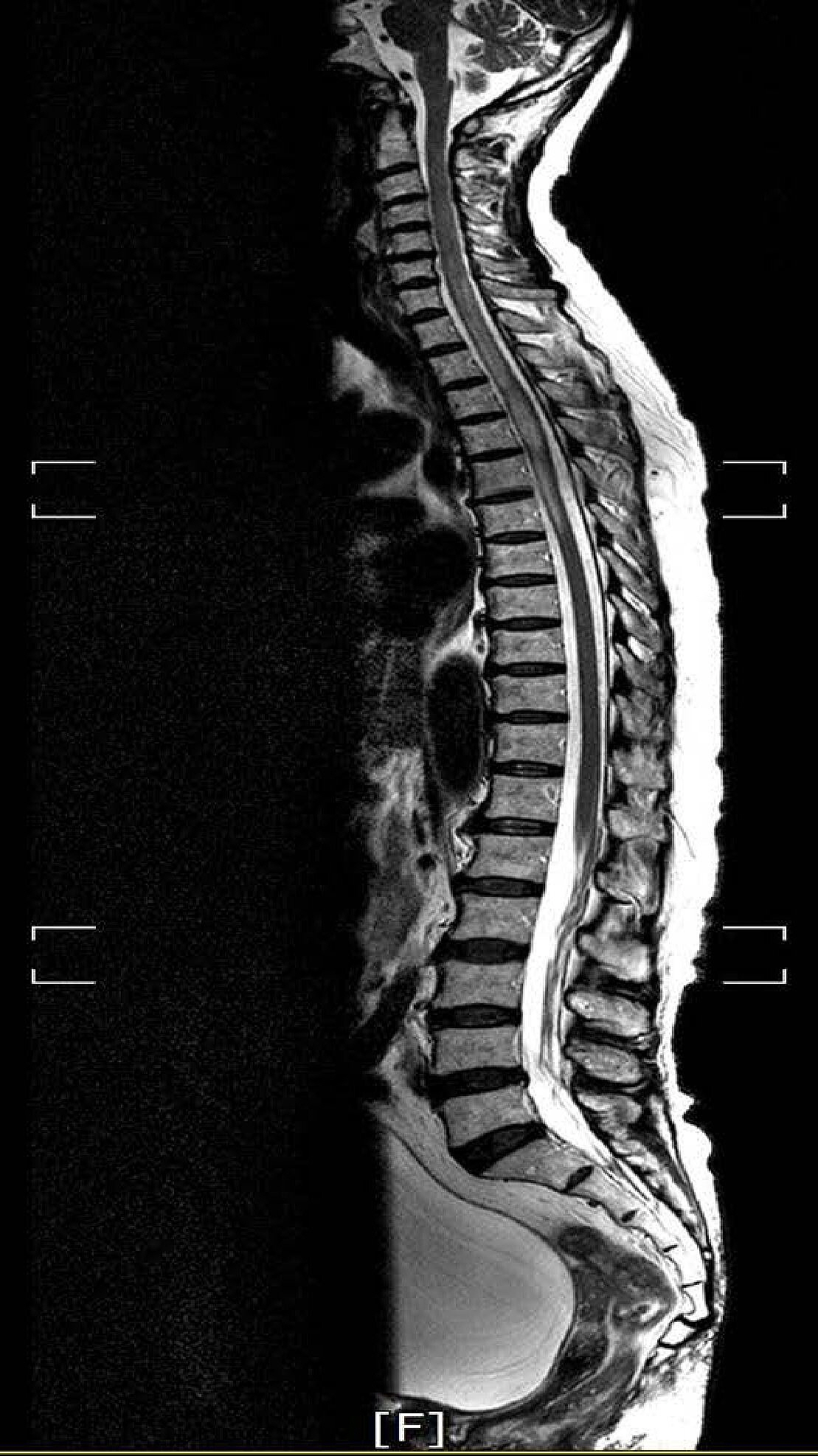
Fig.┬Ā2.
An approximately 35-mm ovoid, well-marginated mass with enhancement in the spinal cord at T3 to T4. (A) Sagittal image, (B) axial image. Written informed consent was obtained for publication of this case report and accompanying images.
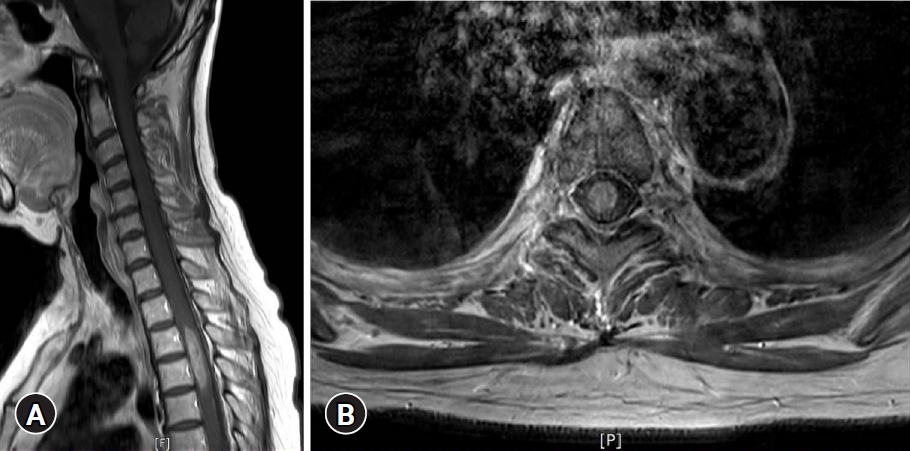
Fig.┬Ā3.
Microscopic image showing a biphasic pattern with cellular Antoni A and hypocellular Antoni B areas (H&E, ├Ś40). Written informed consent was obtained for publication of this case report and accompanying images.
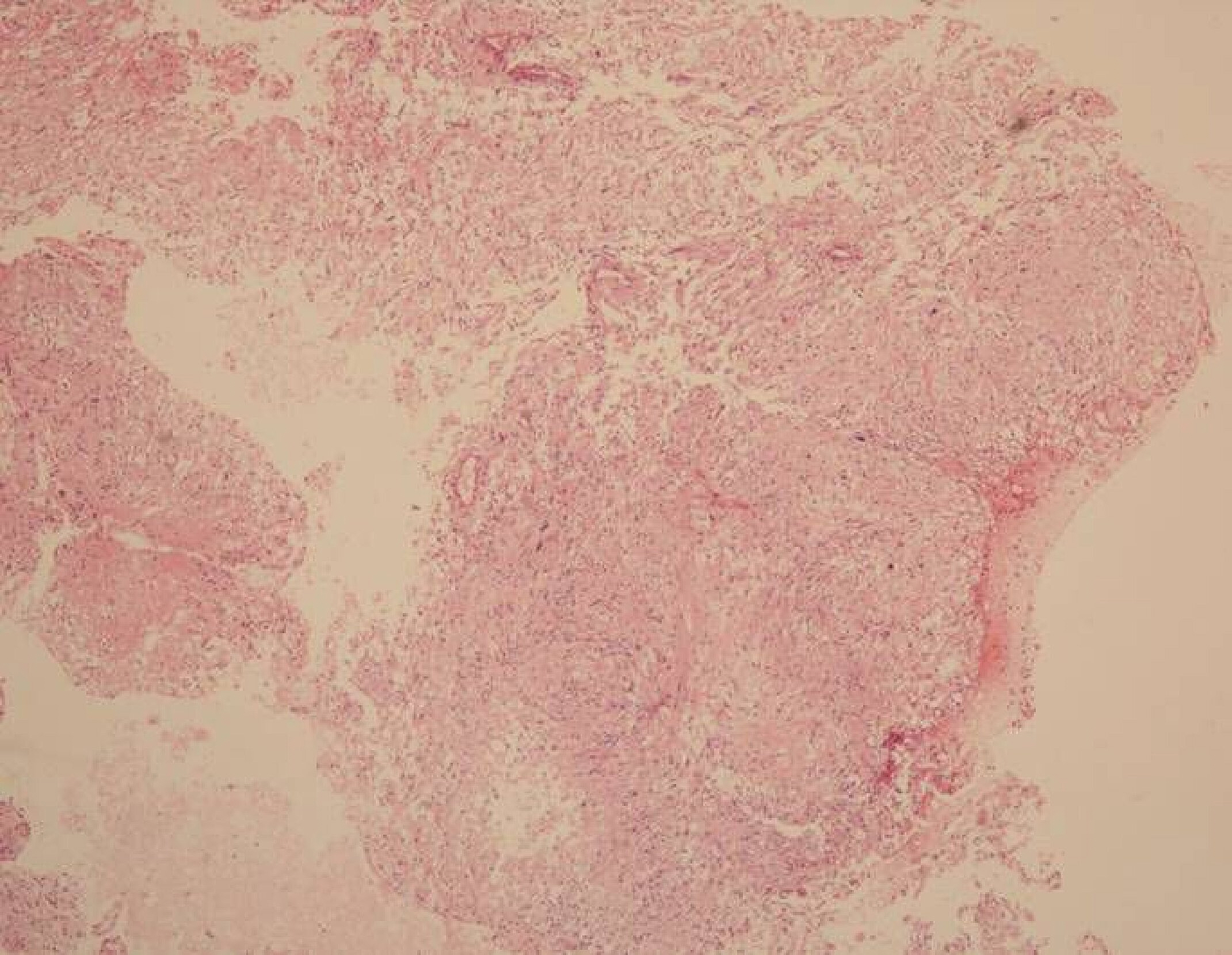
Fig.┬Ā4.
Microscopic image revealing compact fasciles of elongated tumor cells with slight nuclear polymorphism (H&E, ├Ś100). Written informed consent was obtained for publication of this case report and accompanying images.

Fig.┬Ā5.
Immunohistochemical stains demonstrating diffuse positivity for S-100 protein (├Ś100). Written informed consent was obtained for publication of this case report and accompanying images.

REFERENCES
1. Brown KM, Dean A, Sharr MM. Thoracic intramedullary schwannoma. Neuropathol Appl Neurobiol 2002;28:421-4.


3. Penfield W. Notes on operative technic in neurosurgery. Ann Surg 1946;124:383-5.


4. Hayashi F, Sakai T, Sairyo K, et al. Intramedullary schwannoma with calcification of the epiconus. Spine J 2009;9:e19-23.

6. Herregodts P, Vloeberghs M, Schmedding E, Goossens A, Stadnik T, DŌĆÖHaens J. Solitary dorsal intramedullary schwannoma: case report. J Neurosurg 1991;74:816-20.


7. Kim SD, Nakagawa H, Mizuno J, Inoue T. Thoracic subpial intramedullary schwannoma involving a ventral nerve root: a case report and review of the literature. Surg Neurol 2005;63:389-93.


10. Kahilogullari G, Aydin Z, Ayten M, Attar A, Erdem A. Schwannoma of the conus medullaris. J Clin Neurosci 2005;12:80-1.
















 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print